Question: help Use the data below to calculate Gn for the reaction: H2O(g)+CO(g)H2(g)+CO2(g) Data: H2(g)+21O2(g)H2O(g)Grxn=228.6kJ/mol 2CO(g)+O2(g)2CO2(g)Grxn=514.4kJ/mol 1st attempt
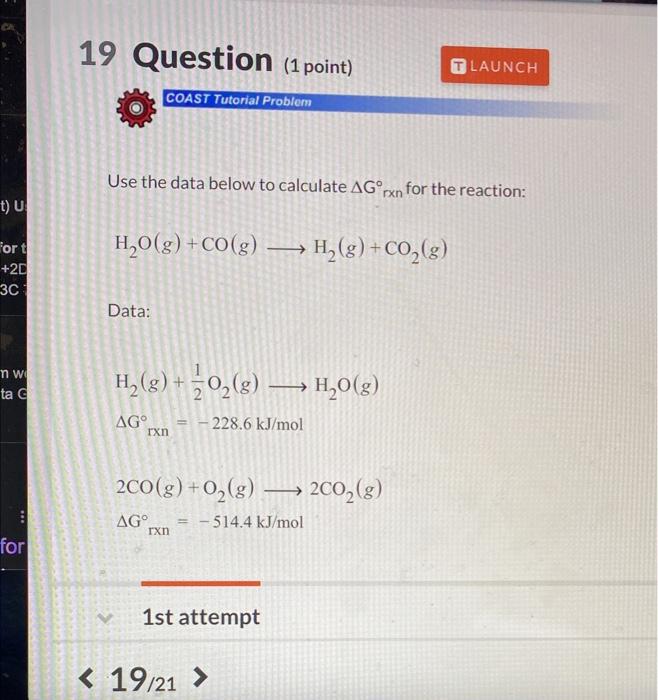
Use the data below to calculate Gn for the reaction: H2O(g)+CO(g)H2(g)+CO2(g) Data: H2(g)+21O2(g)H2O(g)Grxn=228.6kJ/mol 2CO(g)+O2(g)2CO2(g)Grxn=514.4kJ/mol 1st attempt
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


