Question: Help with 2 problems please Dinitrogen tetroxide decomposes to form nitrogen dioxide in a second-order reaction: N2O4(g)2NO2(g) At 400.0K, the rate constant for this reaction

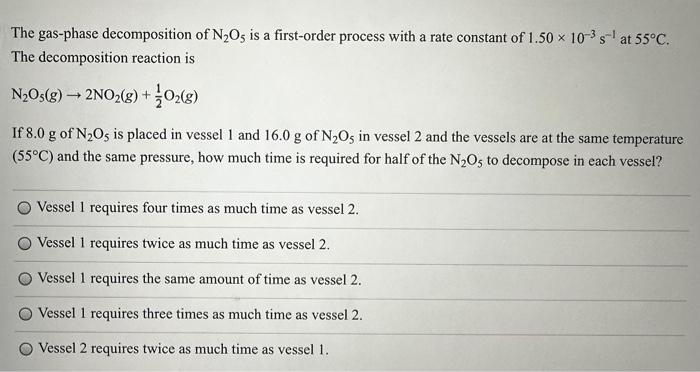
Dinitrogen tetroxide decomposes to form nitrogen dioxide in a second-order reaction: N2O4(g)2NO2(g) At 400.0K, the rate constant for this reaction has been measured to be 2.9108L/(mols). Suppose 0.742mol of N2O4(g) is placed in a sealed 34.3 - L container at 400.0K and allowed to react. What is the total pressure inside the vessel after 49.6ns has elapsed? (R=0.0821(Latm)/(Kmol)) (This question is tricky. You should first the second order equation to calculate the [N2O4] t that remains after 49.6ns (convert ns to sec). Then, you have to remember that you also formed NO2(g) in the reaction. So, you also have to add the pressure from NO2(g) to [N2O4]t to get the total pressure. The answer is not 0.541atm, which is only the pressure from [N2O4]t.) 0.541atm 0.879atm 0.7138atm 0.7319atm 2.13atm The decomposition reaction is N2O5(g)2NO2(g)+21O2(g) If 8.0g of N2O5 is placed in vessel 1 and 16.0g of N2O5 in vessel 2 and the vessels are at the same temperature (55C) and the same pressure, how much time is required for half of the N2O5 to decompose in each vessel? Vessel 1 requires four times as much time as vessel 2. Vessel 1 requires twice as much time as vessel 2. Vessel 1 requires the same amount of time as vessel 2. Vessel 1 requires three times as much time as vessel 2. Vessel 2 requires twice as much time as vessel 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


