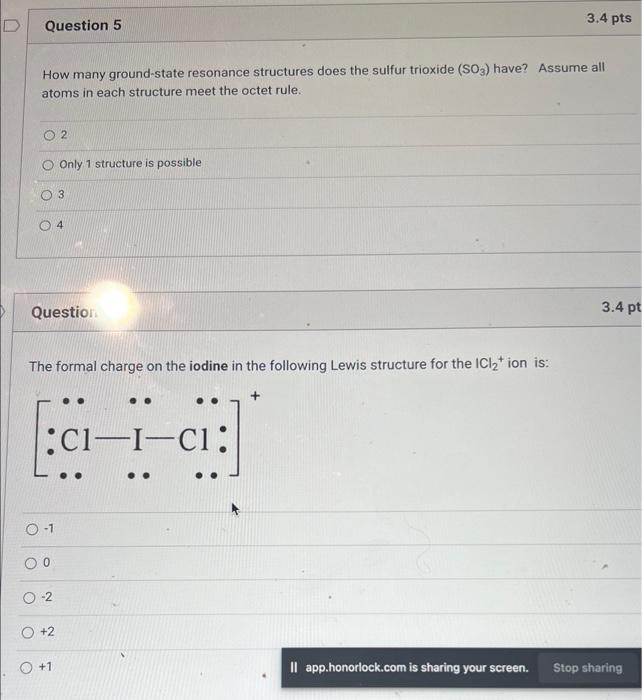
Question: help with 5&6 How many ground-state resonance structures does the sulfur trioxide (SO3) have? Assume all atoms in each structure meet the octet rule. 2
 help with 5&6
help with 5&6How many ground-state resonance structures does the sulfur trioxide (SO3) have? Assume all atoms in each structure meet the octet rule. 2 Only 1 structure is possible 3 4 Question: The formal charge on the iodine in the following Lewis structure for the ICl2+ion is: 1 0 2 +2 +1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


