Question: help with all please You need to make an aqueous solution of 0.238M ammonium acetate for an experiment in lab, using a 250 mL volumetric

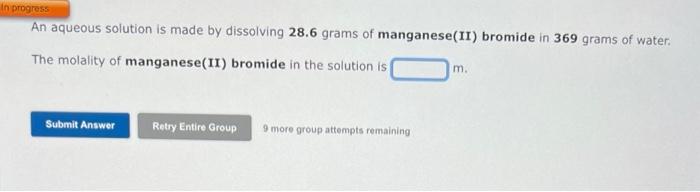
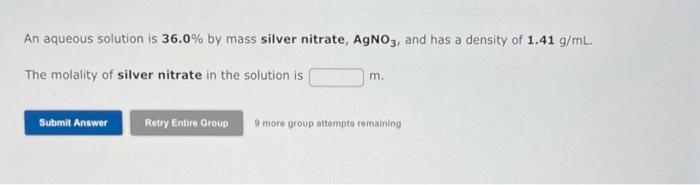
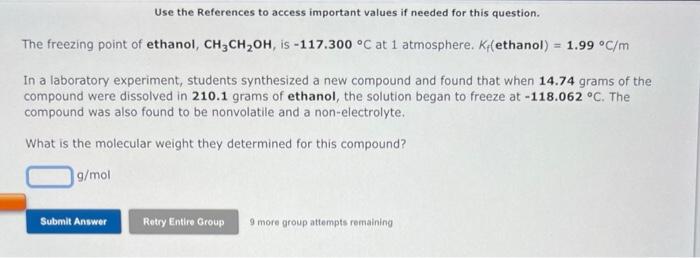
You need to make an aqueous solution of 0.238M ammonium acetate for an experiment in lab, using a 250 mL volumetric flask. How much solid ammonium acetate should you add? grams An aqueous solution is made by dissolving 28.6 grams of manganese(II) bromide in 369 grams of water: The molality of manganese(II) bromide in the solution is 9 more group attempts remaining An aqueous solution is 36.0% by mass silver nitrate, AgNO3, and has a density of 1.41g/mL. The molality of silver nitrate in the solution is m. 9 more group attempts remaining Use the References to access important values if needed for this question. The freezing point of ethanol, CH3CH2OH, is 117.300C at 1 atmosphere. Kf( ethanol) =1.99C/m In a laboratory experiment, students synthesized a new compound and found that when 14.74grams of the compound were dissolved in 210.1 grams of ethanol, the solution began to freeze at 118.062C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


