Question: help with calculations and results portion plzzzzz Data for hydrate sample I begin{tabular}{|l|l|} hline & hline Clean dry test tube & hline Test
help with calculations and results portion plzzzzz
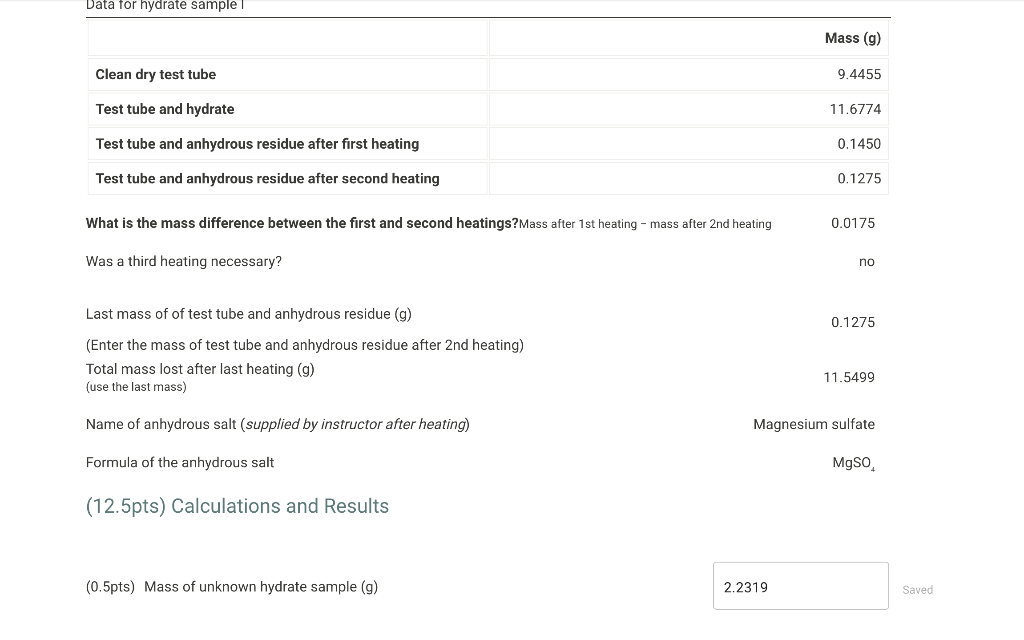
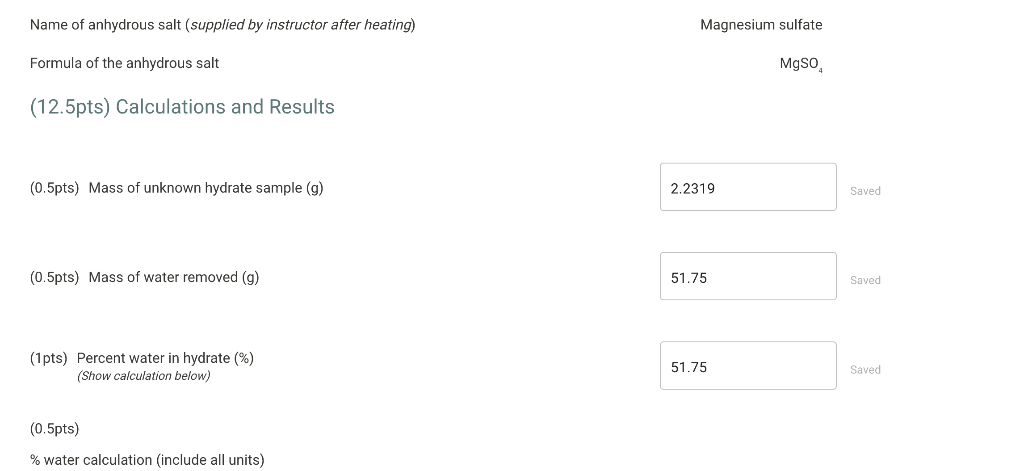
Data for hydrate sample I \begin{tabular}{|l|l|} \hline & \\ \hline Clean dry test tube & \\ \hline Test tube and hydrate & \\ \hline Test tube and anhydrous residue after first heating \\ \hline Test tube and anhydrous residue after second heating & \\ \hline \end{tabular} What is the mass difference between the first and second heatings?Mass after 1 st heating - mass after 2nd heating 0.0175 Was a third heating necessary? no Last mass of of test tube and anhydrous residue (g) (Enter the mass of test tube and anhydrous residue after 2 nd heating) Total mass lost after last heating ( g ) (use the last mass) 11.5499 Name of anhydrous salt (supplied by instructor after heating) Magnesium sulfate Formula of the anhydrous salt MgSO4 (12.5pts) Calculations and Results (0.5pts) Mass of unknown hydrate sample ( g) 2.2319 Saved Name of anhydrous salt (supplied by instructor after heating) Magnesium sulfate Formula of the anhydrous salt MgSO4 (12.5pts) Calculations and Results (0.5pts) Mass of unknown hydrate sample ( g) Saved (0.5pts) Mass of water removed ( g) Saved (1pts) Percent water in hydrate (\%) (Show calculation below) Saved (0.5pts) % water calculation (include all units)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


