Question: help with e ,f and g please. 3.1 Assuming the validity of Raoult's law, do the following calculations for the benzene(1)/toluene(2) system: (a) Given x1=0.33
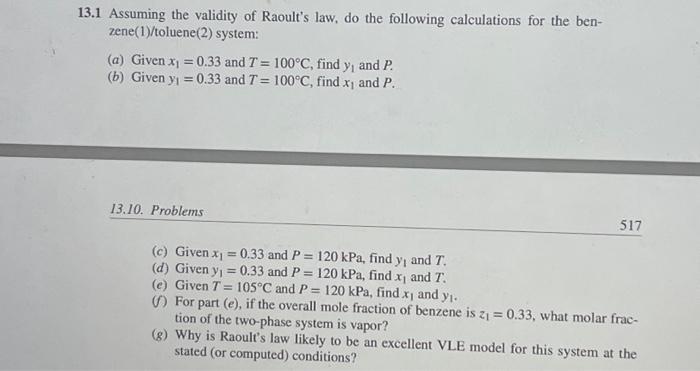
3.1 Assuming the validity of Raoult's law, do the following calculations for the benzene(1)/toluene(2) system: (a) Given x1=0.33 and T=100C, find y1 and P. (b) Given y1=0.33 and T=100C, find x1 and P. 13.10. Problems 517 (c) Given x1=0.33 and P=120kPa, find y1 and T. (d) Given y1=0.33 and P=120kPa, find x1 and T. (c) Given T=105C and P=120kPa, find x1 and y1. (f) For part (e), if the overall mole fraction of benzene is z1=0.33, what molar fraction of the two-phase system is vapor? (g) Why is Raoult's law likely to be an excellent VLE model for this system at the stated (or computed) conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


