Question: help with empty boxes please need help with molarity questions Molar mass determination of ethylene glycol: cooling curve for pure water Jllow the directions on




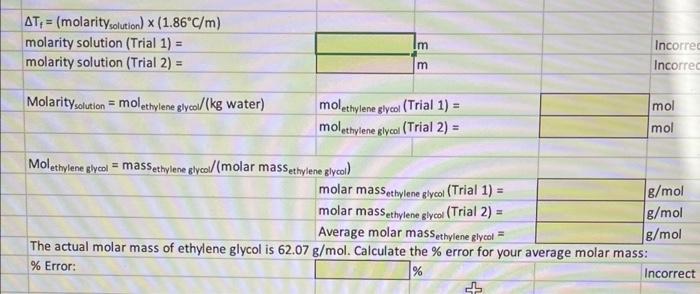
Molar mass determination of ethylene glycol: cooling curve for pure water Jllow the directions on the first PUREWATER graphs on the graph tab to get the freezing points of water for each trial Perparation ice/Saltwater bath Mass NaCl(g) : Temperature of ice/saltwater bath: \begin{tabular}{|r|l|} \hline 49.36 & g \\ \hline15 & C \\ \hline \end{tabular} Molar mass determination of ethylene glycol: cooling curve for pure water Mass of beaker + culture tube: 174.88g The actual molar mass of ethylene glycol is 62.07g/mol. Calculate the % error for your average molar mass: \% Error: Incorrect Freezing Point Depression: Determination of the Molar Mass of Ethylene Glycol Prepare ice/saltwater bath Determine the mass of NaCl necessary to lower freezing point of 300mL of distilled water to 10C Mass NaCl needed: 49.36g Based on the actual mass of NaCl you used in #1, determine the theoretical freezing point of the ice/saltwate Theoretical freezing point: Determine the \% error for the temperature of your ice/saltwater bath (comparing the temperature you measured, \#3, and the theoretical freezing point, \#14): \% Error: 50% Molar mass determination of ethylene glycol: cooling curve for pure water w the directions on the first PURE WATER graphs on the graph tab to get the freezing points of water for each trial \begin{tabular}{l|l|r|} \hline Freezing point of water: & Trial 1: & 0.024 \\ & Trial 2: & 0 \\ \hline \end{tabular} Determine the % error for the freezing point of water (comparing the average temperature you determined in \#17 and the actual freezing point; Note: use Kelvin): \% Error: Molar mass determination of ethylene glycol: cooling cureve for aqueous ethylene glycol solution W the directions on the first ETHYLENE GLYCOL graphs on the graph tab to get the freezing points of water for each \begin{tabular}{l|l|r|} \hline Freezing point of solution: & Trial 1: & 0.72 \\ \hline & Trial 2: & 9.4 \\ \hline \end{tabular} Determination of molar mass of ethylene glycol calculations: Tf=Tt solution Tt water = (these should be positive) Tf=( molarity solution )(1.86C/m) The actual molar mass of ethylene glycol is 62.07g/mol. Calculate the \% error for your average molar mass: \% Error: % Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


