Question: *HELP WITH POSTLAB EXERCISE For each solvent Alcohol, Alkanes, 2-propanol and acetone, 1-butanol, and n-pentane use the information obtained to concisely explain variations in the
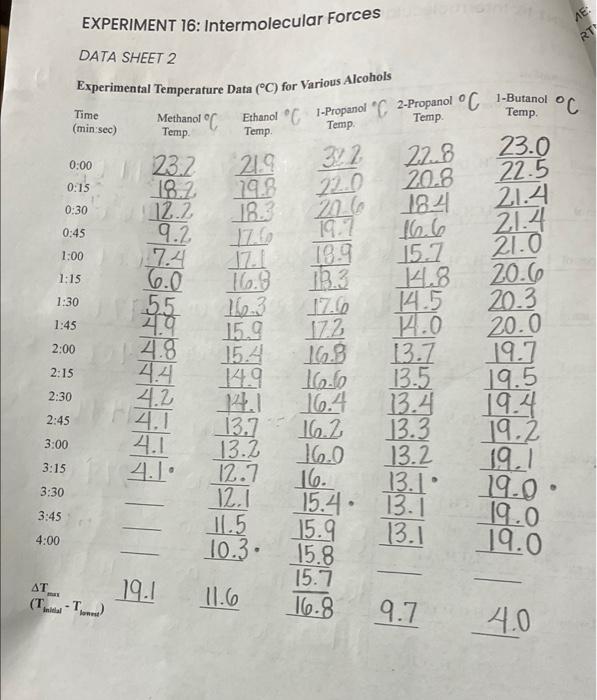
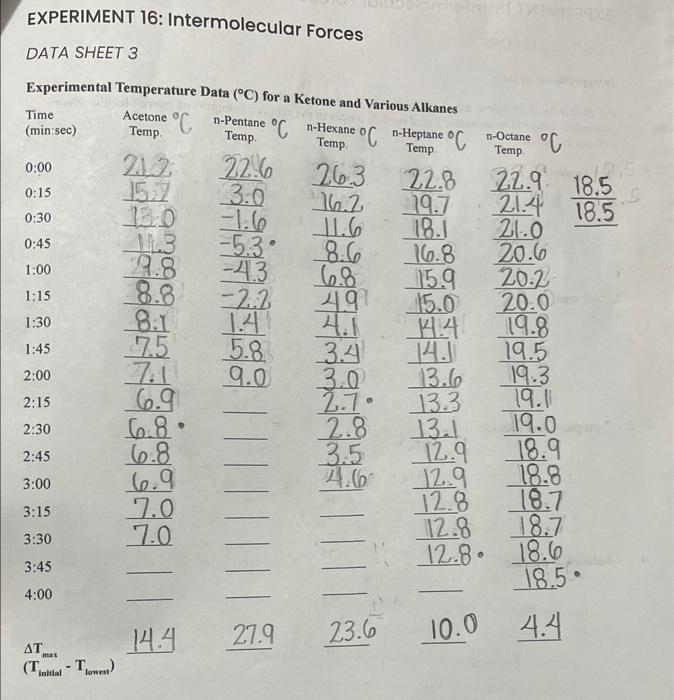

EXPERIMENT 16: Intermolecular Forces DATA SHEET 2 Experimental Temperature Data (C) for a Ketone and EXPERIMENT 16: Intermolecular Forces POSTLAB EXERCISE Interpretation of Data For each solvent set below, use the information you have obtained to concisely explain variations in the maximum change in temperature based on differences in the relative strengths of intermolecular forces within a given set. 1. Alcohols: 2. Alkanes: 3. 2-propanol and acetone: 4. 1-butanol, n-pentane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


