Question: 3. Van der Waals and Virial constants for N are given below. Calculate values for B at 103 K and 273 K and C
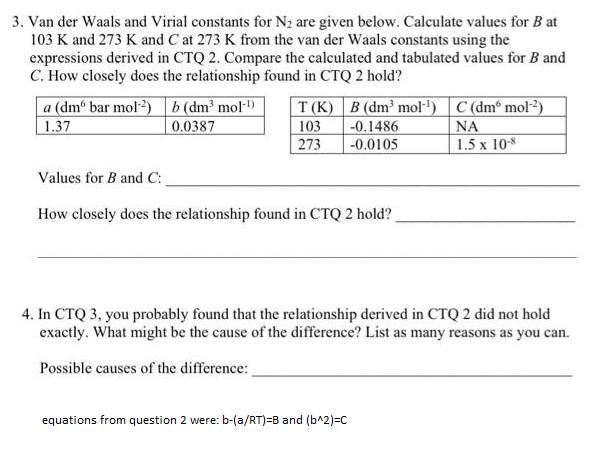
3. Van der Waals and Virial constants for N are given below. Calculate values for B at 103 K and 273 K and C at 273 K from the van der Waals constants using the expressions derived in CTQ 2. Compare the calculated and tabulated values for B and C. How closely does the relationship found in CTQ 2 hold? a (dm bar mol-2) b (dm mol-) 1.37 0.0387 T(K) 103 273 B (dm mol-) C (dm mol) -0.1486 -0.0105 1.5 x 10-8 Values for B and C: How closely does the relationship found in CTQ 2 hold? 4. In CTQ 3, you probably found that the relationship derived in CTQ 2 did not hold exactly. What might be the cause of the difference? List as many reasons as you can. Possible causes of the difference: equations from question 2 were: b-(a/RT)=B and (b^2)=C
Step by Step Solution
3.50 Rating (160 Votes )
There are 3 Steps involved in it
3 Values of B and C are given in the image uploaded How closely the relationship found in CTQ2 hold ... View full answer

Get step-by-step solutions from verified subject matter experts


