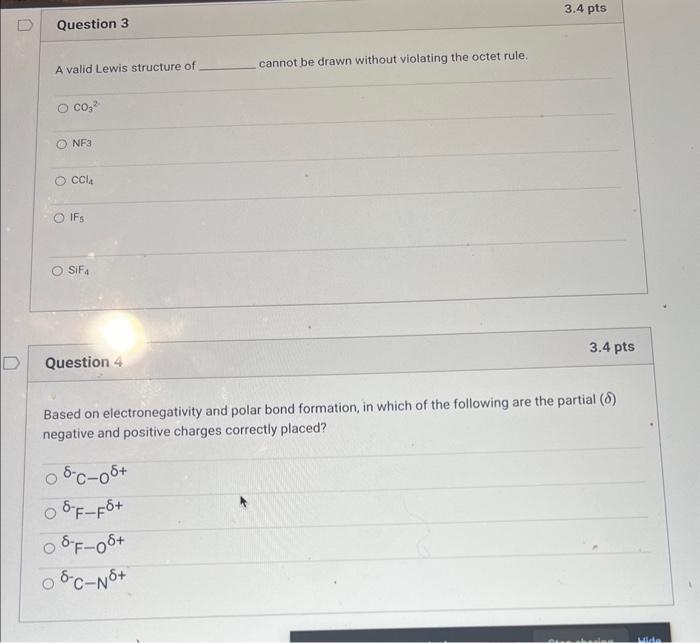
Question: help with question 3&4 A valid Lewis structure of cannot be drawn without violating the octet rule. CO32 NF3 CCl4 Question 4 3.4 pts Based
 help with question 3&4
help with question 3&4A valid Lewis structure of cannot be drawn without violating the octet rule. CO32 NF3 CCl4 Question 4 3.4 pts Based on electronegativity and polar bond formation, in which of the following are the partial () negative and positive charges correctly placed? 8CO+FF+O+=N+
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


