Question: help with resonace 7. A) Draw all CRS and only CRS of the following molecule. Non-contributing RS might appear on your scratch paper as vou
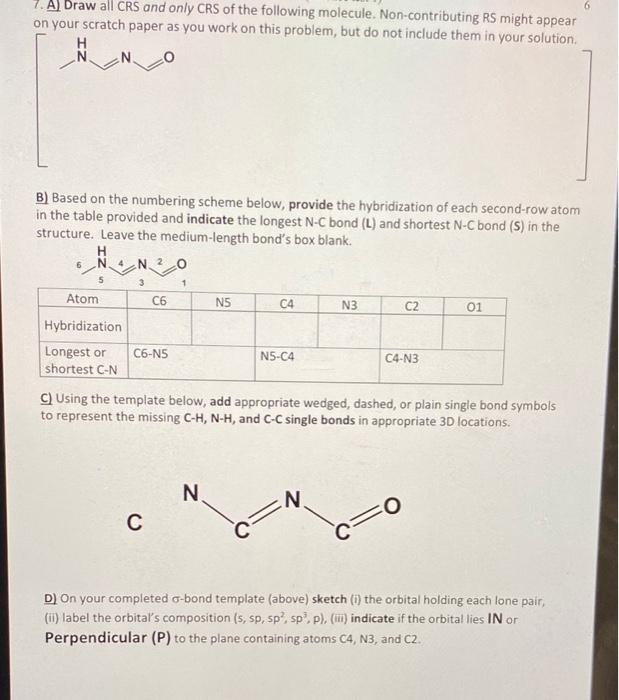
7. A) Draw all CRS and only CRS of the following molecule. Non-contributing RS might appear on your scratch paper as vou work on this problem, but do not include them in your solution. B) Based on the numbering scheme below, provide the hybridization of each second-row atom in the table provided and indicate the longest N-C bond (L) and shortest N-C bond (S) in the structure. Leave the medium-length bond's box blank. C) Using the template below, add appropriate wedged, dashed, or plain single bond symbols to represent the missing C-H, N-H, and C-C single bonds in appropriate 3 D locations. D) On your completed -bond template (above) sketch (i) the orbital holding each lone pair, (ii) label the orbital's composition (s, sp,5p2,sp3,p), (iii) indicate if the orbital lies IN or Perpendicular (P) to the plane containing atoms C4,N3, and C2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


