Question: Help with table 2 and the second column of table 3. How do I assign the modes? And what are the accepted values of K1
 Help with table 2 and the second column of table 3. How do I assign the modes? And what are the accepted values of K1 ect..
Help with table 2 and the second column of table 3. How do I assign the modes? And what are the accepted values of K1 ect..
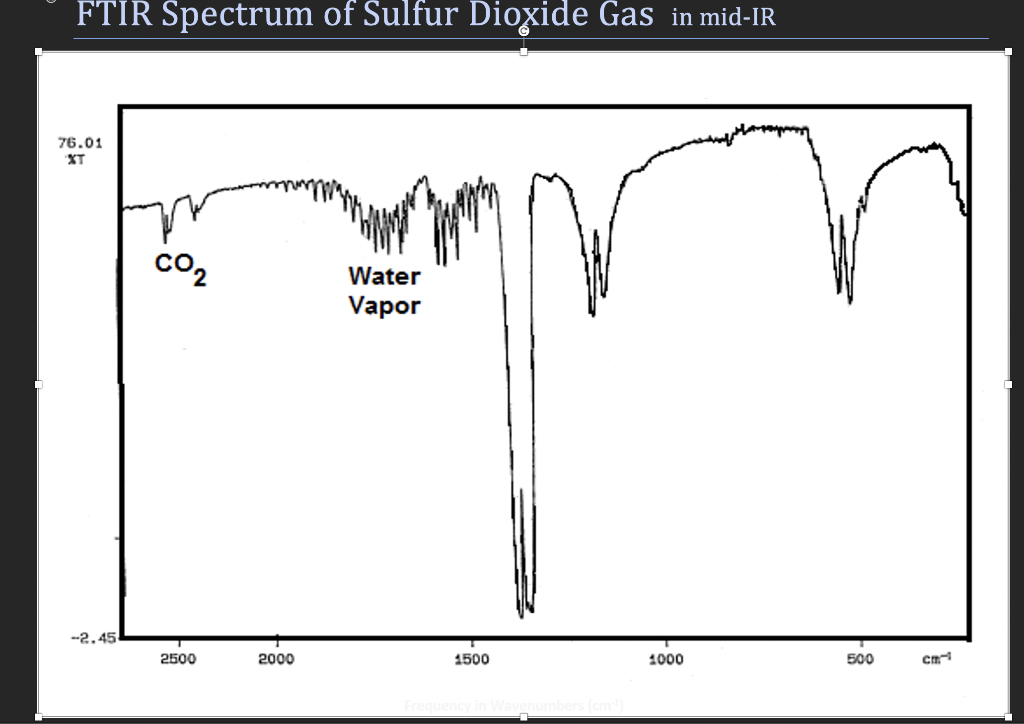
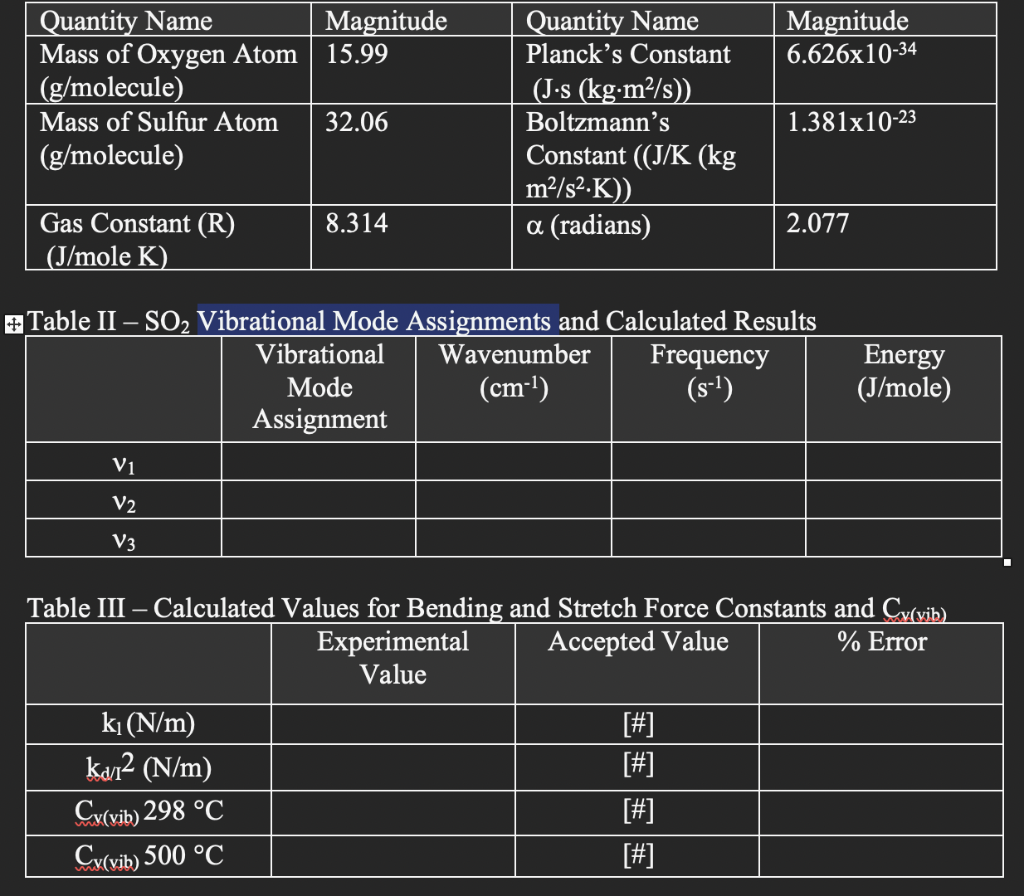
FTIR Spectrum of Sulfur Dioxide Gas in mid-IR 76.01 Com Water Vapor -2.45 2500 2000 1500 1000 500 Magnitude 15.99 Magnitude 6.626x10-34 Quantity Name Mass of Oxygen Atom (g/molecule) Mass of Sulfur Atom (g/molecule) 32.06 Quantity Name Planck's Constant (J-s (kg.m2/s) Boltzmann's Constant ((J/K (kg m2/s2.K)) a (radians) 1.381x10-23 8.314 2.077 Gas Constant (R) (J/mole K) #Table II SO2 Vibrational Mode Assignments and Calculated Results Vibrational Wavenumber Frequency Mode (cm-1) (s-1) Assignment Energy (J/mole) Vi V2 V3 - Table III Calculated Values for Bending and Stretch Force Constants and Cy(vib) Experimental Accepted Value % Error Value ki(N/m) [#] kal2 (N/m) [#] Cy(vib) 298 C [#] Cy(vib) 500 C [#]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


