Question: help with wrong ones please. last attempt (3pts) Titrating a Polyprotic Acid Mole to Mole ratios Given the following generic equations: a. g of acid
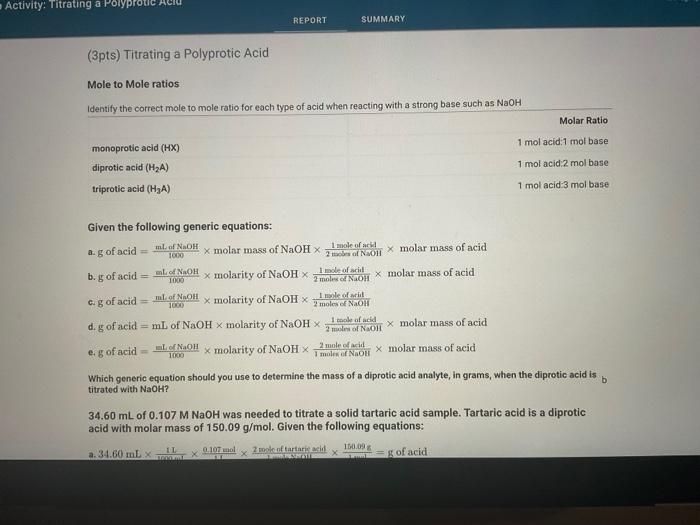
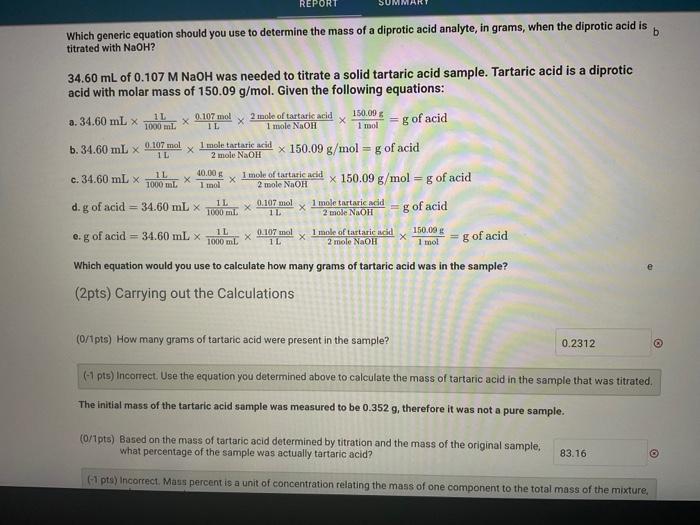
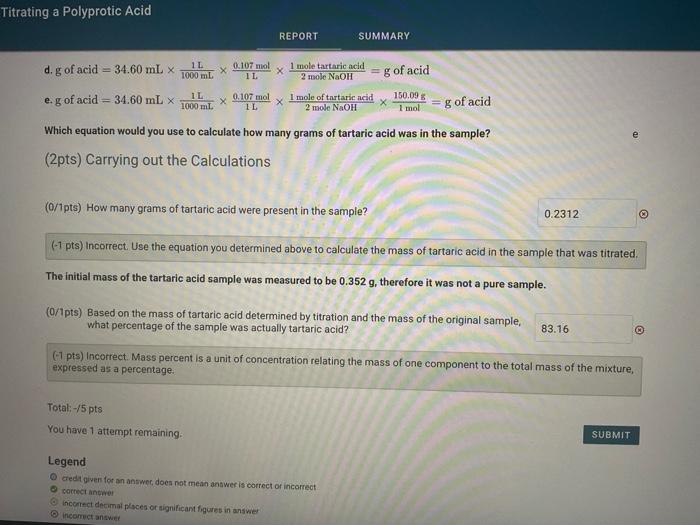
(3pts) Titrating a Polyprotic Acid Mole to Mole ratios Given the following generic equations: a. g of acid =1000mlofNaOH molar mass of NaOH2malaolNaOH1moleofarid molar mass of acid b. g of acid =1000aLafNaOH molarity of NaOH2molesNaOH1moleofacil molar mass of acid c. g of acid =1600mlofNaOH molarity of NaOH2molecofNaOHI1moleofarit d. g of acid =mL of NaOH molarity of NaOH2mudnofNaOH1makafadia molar mass of acid e. g of acid =1000e.ofNaOH molarity of NaOH 1molehorNaOH2moleofarid molar mass of acid Which generic equation should you use to determine the mass of a diprotic acid analyte, in grams, when the diprotic acid is b titrated with NaOH ? 34.60 mL of 0.107MNaOH was needed to titrate a solid tartaric acid sample. Tartaric acid is a diprotic acid with molar mass of 150.09g/mol. Given the following equations: Which generic equation should you use to determine the mass of a diprotic acid analyte, in grams, when the diprotic acid is titrated with NaOH ? 34.60 mL of 0.107MNaOH was needed to titrate a solid tartaric acid sample. Tartaric acid is a diprotic acid with molar mass of 150.09g/mol. Given the following equations: a. 34.60mL1000mL1L1L0.107mol1moleNaOH2moleoftartaricacid1mol150.09g=g of acid b. 34.60mL1L0.107mol2moleNaOH1moletartaricacid150.09g/mol=g of acid c. 34.60mL1000mL1L1mol40.00g2moleNaOH1moleoftartaricadl150.09g/mol=g of acid d. g of acid =34.60mL1000mL1L1L0.107mol2modeNaOH1moletartaricacid=g of acid e. g of acid =34.60mL1000mL1L1L0.07mol2moleNaOH1moleoftartaricacid1mol150.09g=g of acid Which equation would you use to calculate how many grams of tartaric acid was in the sample? (2pts) Carrying out the Calculations (O/1pts) How many grams of tartaric acid were present in the sample? (-1 pts) Incorrect. Use the equation you determined above to calculate the mass of tartaric acid in the sample that was titrated. The initial mass of the tartaric acid sample was measured to be 0.352g, therefore it was not a pure sample. (0/1pts) Based on the mass of tartaric acid determined by titration and the mass of the original sample, what percentage of the sample was actually tartaric acid? (-7 pts) incorrect. Maes percent is a unit of concentration relating the mass of one component to the total mass of the mixture. Titrating a Polyprotic Acid d. g of acid =34.60mL1000mL1L1L0.107mol2mioleNaOH1moletarturicacid=g of acid e. g of acid =34.60mL1000mL1L1L0.107mol2moleNaOH1moleoftartaricacid1mol150.09g=g of acid Which equation would you use to calculate how many grams of tartaric acid was in the sample? (2pts) Carrying out the Calculations (0/1pts) How many grams of tartaric acid were present in the sample? (-1 pts) Incorrect. Use the equation you determined above to calculate the mass of tartaric acid in the sample that was titrated. The initial mass of the tartaric acid sample was measured to be 0.352g, therefore it was not a pure sample. (0/1pts) Based on the mass of tartaric acid determined by titration and the mass of the original sample, what percentage of the sample was actually tartaric acid? (-1 pts) Incorrect. Mass percent is a unit of concentration relating the mass of one component to the total mass of the mixture, expressed as a percentage
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


