Question: Consider the Following Molecule: HN- Px NH a) Determine the reducible representation of the SALC group orbitals formed from the p, atomic orbitals of
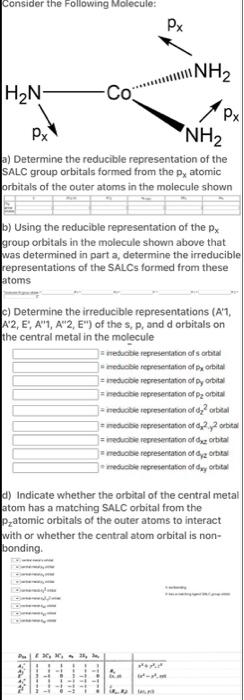
Consider the Following Molecule: HN- Px NH a) Determine the reducible representation of the SALC group orbitals formed from the p, atomic orbitals of the outer atoms in the molecule shown atoms Co b) Using the reducible representation of the px group orbitals in the molecule shown above that was determined in part a, determine the irreducible representations of the SALCs formed from these P3, 32, 3 Fata Px c) Determine the irreducible representations (A'1, A2, E, A1, A2, E") of the s, p, and d orbitals on the central metal in the molecule NH d) Indicate whether the orbital of the central metal atom has a matching SALC orbital from the Patomic orbitals of the outer atoms to interact with or whether the central atom orbital is non- bonding. Px meducible representation of s orbital meducible representation of px orbital =reducible representation of py orbital meducible representation of p orbital ineducible representation of d orbital meducible representation of d2.2 orbital imeducible representation of de orbital meducible representation of dy orbital meducible representation of day orbital 6-35,00 JALAR
Step by Step Solution
3.55 Rating (148 Votes )
There are 3 Steps involved in it
b HN PX NH We de les mine the reducible representation of the SALC group zbitels frund from t... View full answer

Get step-by-step solutions from verified subject matter experts


