Question: Here I have all the information and I need help with the questions. Please and Thank you. Data Part A-Salt Solutions 20.5C Water Temperature: Mass
Here I have all the information and I need help with the questions. Please and Thank you.
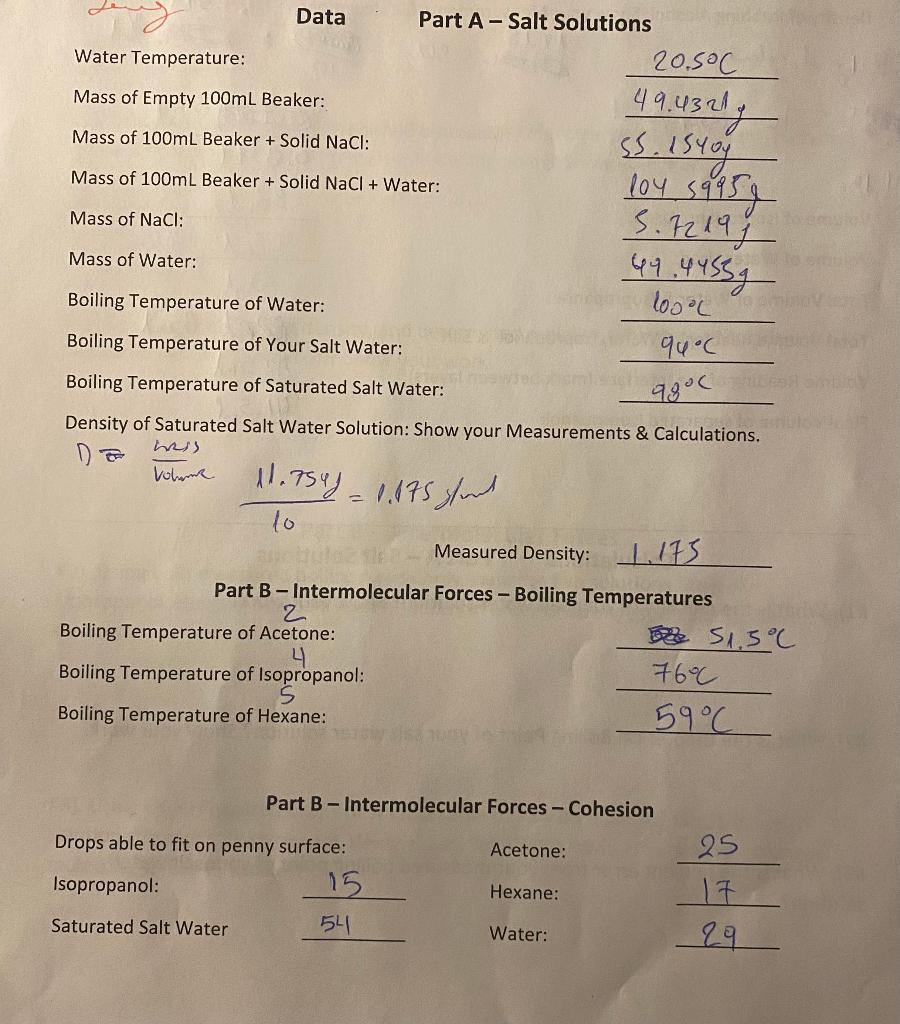



Data Part A-Salt Solutions 20.5C Water Temperature: Mass of Empty 100mL Beaker: Mass of 100mL Beaker + Solid NaCl: Mass of 100mL Beaker + Solid NaCl + Water: 49.43 217 SS. istog 104 59959 44.44532 Mass of NaCl: 3.72198 Mass of Water: Boiling Temperature of Water: 1o0oC 94C Boiling Temperature of Your Salt Water: Boiling Temperature of Saturated Salt Water: 9806 Density of Saturated Salt Water Solution: Show your Measurements & Calculations. Do nis Vohne 11.754)- 1.175 soud to Measured Density: 1.173 Part B - Intermolecular Forces - Boiling Temperatures 2 Boiling Temperature of Acetone: e 51.3C 4 Boiling Temperature of Isopropanol: 7600 Boiling Temperature of Hexane: 59C Part B - Intermolecular Forces - Cohesion Acetone: Drops able to fit on penny surface: Isopropanol: 15 Saturated Salt Water 541 25 17 Hexane: ale Water: 29 Boiling Point Elevation Part C-Miscible Solvents 0.8.379 4.18 - S.8014 g/ml 14.189 Density of Rubbing Alcohol: Show your Measurements & Calculations 5. bolz of eglender. with isopropanel Measured Density: 0.8379 Volume of isopropanol Used: 30nd Volume of Water Used: 4 rent Total Volume of Water & Isopropanol: Bond Total Volume Reading of Water, Isopropanol & Salt: B 85ml Volume Reading of the Interface (mark between layers): 43.5ml Final Volume of separated Isopropanol: yl Sul Calculations Part A-Salt Solutions #1). What is the molality of your salt water solution? Show your work? #2). What is the theoretical Boiling Point of your salt water solution? Show your work. #3). What is the percent error from your measured boiling point of your salt water solution? Page 2 Boiling Point Elevation #4). If you were to use this same mass of Aluminum Chloride, what would be the boiling point? Show your work. #5). Using your boiling temperature of water as a reference point, what is the molality of the Saturated Salt Water based on its boiling point? Show your work. #6). Using your measured density, and determined molality, what is the Molarity of Saturated Salt water solution? Show your work. Part B -Intermolecular Forces #7). Using your measured boiling points only, rank the five solutions (Acetone, Isopropanol, Hexane, Sat. Salt Water, Water) in order of strongest molecular forces to weakest. #8). Using your measured drops of solution able to stay on a penny, rank the five solutions (Acetone, Isopropanol, Hexane, Sat. Salt Water, Water) in order of strongest molecular forces to weakest. Page 3 1 Boiling Point Elevation #9). Of the four main types of intermolecular forces (Dispersion Force, Dipole-Dipole Force, Hydrogen Bonding, lon-Dipole Force), indicate which forces are found in each of the tested materials. Acetone - Isopropanol - Hexane - Saturated Salt Water - Water- #10). Using your data, what is the order of intermolecular forces from strongest to weakest? Explain your choice and refer to your collected data to prove your listed ranking Part C-Miscible Solvents #11). After the liquids separate, what is the top liquid? #12). After the liquids separate, what is the Bottom liquid? #13). What is the percent of isopropanol recovered through this separation? Show your work. #14). Why would adding salt cause the liquids to separate? Page 4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


