Question: .. Here is a cylinder with a piston and electrical heat. the cylinder is insulated. atmospheric pressure = 1 bar mass of piston = 45kg
..
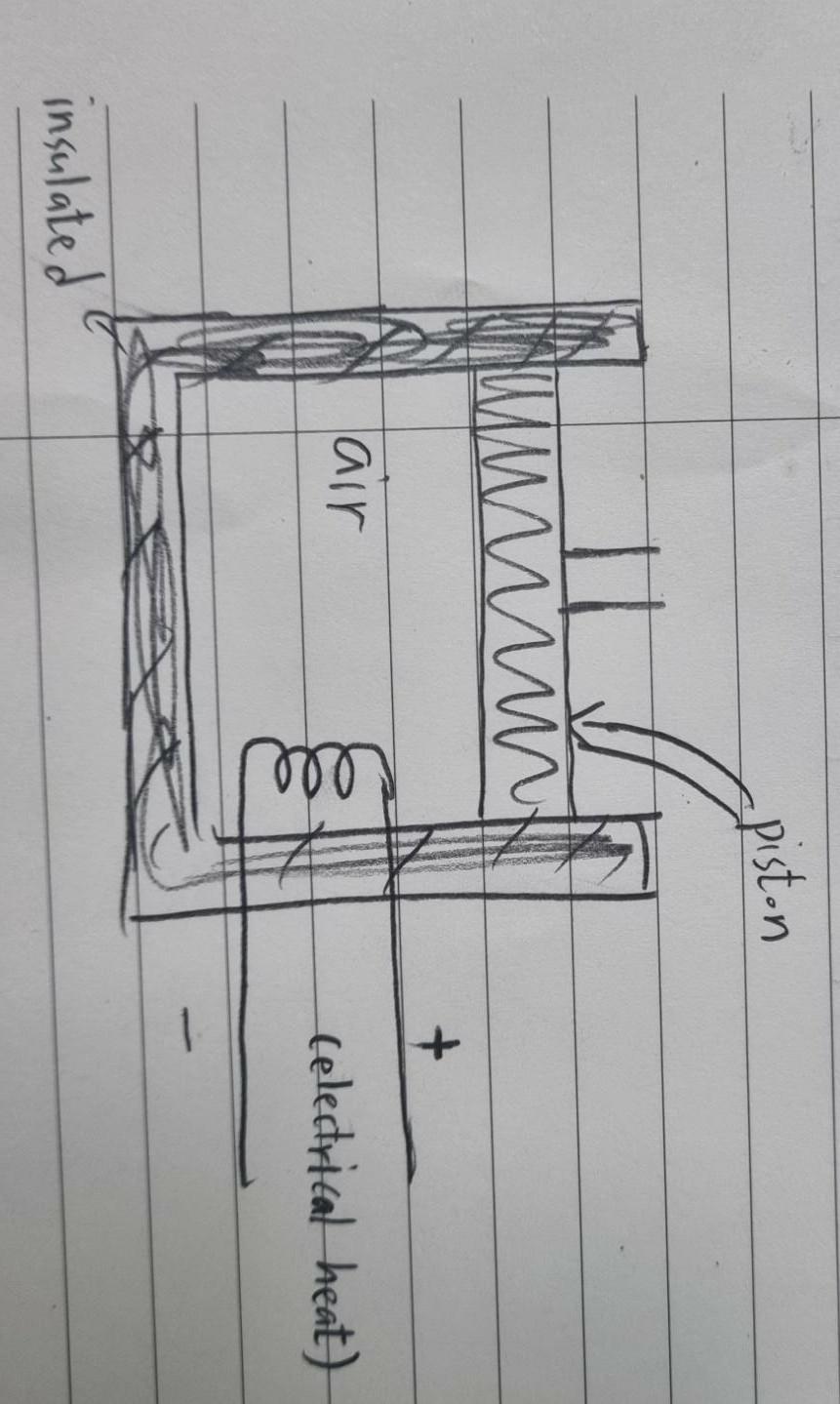
Here is a cylinder with a piston and electrical heat. the cylinder is insulated.
atmospheric pressure = 1 bar
mass of piston = 45kg
Area of piston = 0.09 m^2
mass of air = 0.27kg
(air) The amount of change in volume is 0.045m^3 where the amount of change in internal energy is 42kJ/kg.
How much is work in this system?(Calculate the work at a and b respectively)
a) Suppose that system has air only. (W = ??)
b) Suppose that system has air and piston. ( W = ??)
piston WM ww + lair ell celedrical heat) insulated
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


