Question: here is all the context, i only need part c done please Correct Correct answer is shown. Your answer 179.07 torr was either rounded differently
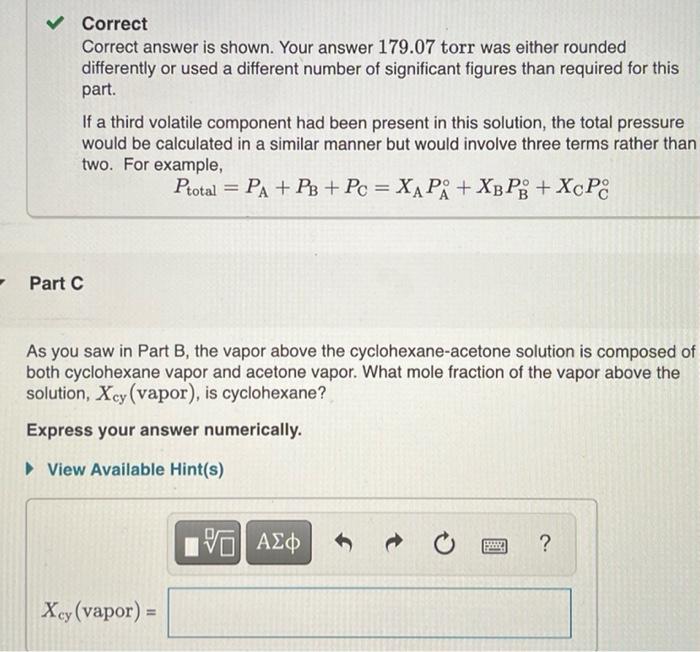
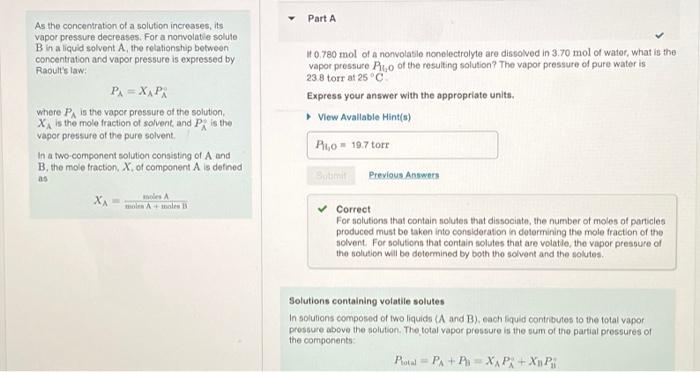
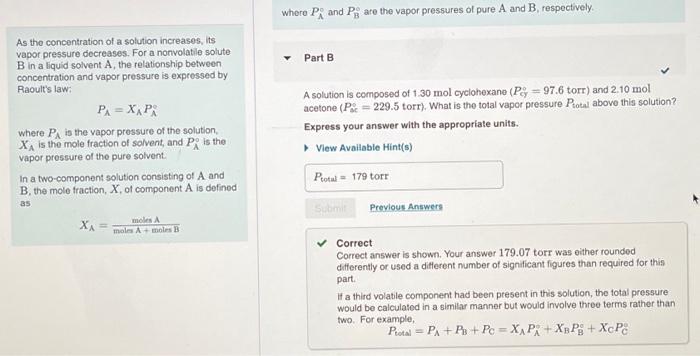
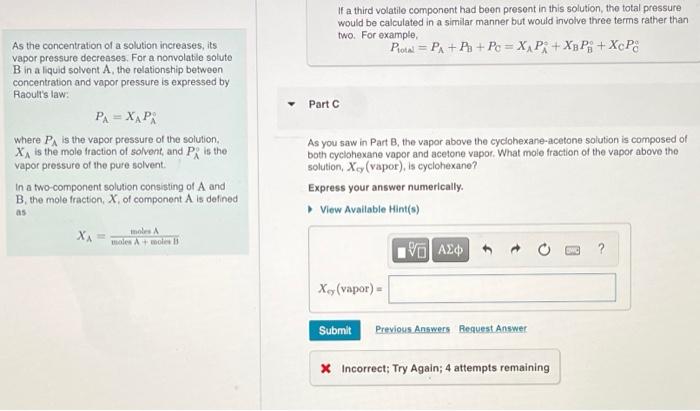
Correct Correct answer is shown. Your answer 179.07 torr was either rounded differently or used a different number of significant figures than required for this part. If a third volatile component had been present in this solution, the total pressure would be calculated in a similar manner but would involve three terms rather than two. For example, Ptotal = PA + P3 + Pc = XA P + X3 PB + XcP8 a Part C As you saw in Part B, the vapor above the cyclohexane-acetone solution is composed of both cyclohexane vapor and acetone vapor. What mole fraction of the vapor above the solution, Xcy (vapor), is cyclohexane? Express your answer numerically. View Available Hint(s) VO AS ] t ? Xcy(vapor) = Part A As the concentration of a solution increases, its vapor pressure decreases. For a nonvolati e soluto B in aliquid solvent A, the relationship between concentration and vapor pressure is expressed by Raoult's law P=X, Pi where PA is the vapor pressure of the solution XA is the mole fraction of solvent and P, is the vapor pressure of the pure solvent In a two-component solution consisting of A and B. the mole fraction. X of component Als defined 0.780 mol of a nonvolatilo nonolectrolyte are dissolved in 3.70 mol of water, what is the vapor pressure Pro of the resulting solution? The vapor pressure of pure water is 23.8 torrat 25C Express your answer with the appropriate units, View Available Hint(s) Rio 19.7 torr as dintre Previous Answers XA ws Amal Correct For solutions that contain solutos that dissociate, the number of moles of particles produced must be taken into consideration in determining the mole fraction of the solvent For solutions that contain solutes that are volatie, the vapor pressure of the solution will be determined by both the solvent and the soluto Solutions containing votatile solutes In solutions composed of two liquids (A and Beach liquid contributes to the total vapor prosture above the solution. The total vapor pressure is the sum of the partial pressures of the components Pol PA+ PixAP+XnP whore P, and P, are the vapor pressures of pure A and B, respectively. Part B As the concentration of a solution increases, its vapor pressure decreases. For a nonvolatile solute B in a liquid solvent A, the relationship between concentration and vapor pressure is expressed by Raoult's law PA=XAPR where in the vapor pressure of the solution XA is the mole fraction of solvent, and Pis the vapor pressure of the pure solvent. In a two-component solution consisting of A and B, the mole fraction, X, of component A is defined as A solution is composed of 1.30 mol cyclohexano (P; = 97.6 torr) and 2.10 mol acetone (P = 229.5 torr). What is the total vapor pressure Potal above this solution? Express your answer with the appropriate units. View Available Hint(s) Protw = 179 torr Previous Answers XA= moles moles Amole B Correct Correct answer is shown. Your answer 179.07 torr was either rounded differently or used a different number of significant figures than required for this part If a third volatile component had been present in this solution, the total pressure would be calculated in a similar manner but would involve three terms rather than two. For example, Pita = PA + P + Pc = XAP: + Xp P9+ XcP If a third volatilo component had been present in this solution, the total pressure would be calculated in a similar manner but would involve three terms rather than two. For example, Protn = PA + P + Pc = X PA + Xy Pi + XcP8 Part As the concentration of a solution increases, its vapor pressure decreases. For a nonvolatile soluto B in aliquid solvent A the relationship between concentration and vapor pressure is expressed by Raoult's law PA=XAPA where is the vapor pressure of the solution, X is the mole fraction of solvent and PR is the vapor pressure of the pure solvent In a two component solution consisting of A and B, the mole fraction of component A is defined as As you saw in Part B, the vapor above the cyclohexane-acetone solution is composed of both cyclohexano vapor and acetone vapor. What mole fraction of the vapor above the solution, X, (vapor), is cyclohexano? Express your answer numerically. View Available Hint(s) XA mos was A V AC ? X. (vapor) Submit Previous Answers Resuest Answer X Incorrect; Try Again; 4 attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


