Question: here is the data just in case please answer all five questions from the last picture with the black background Molar Mass Determination bv Dumas
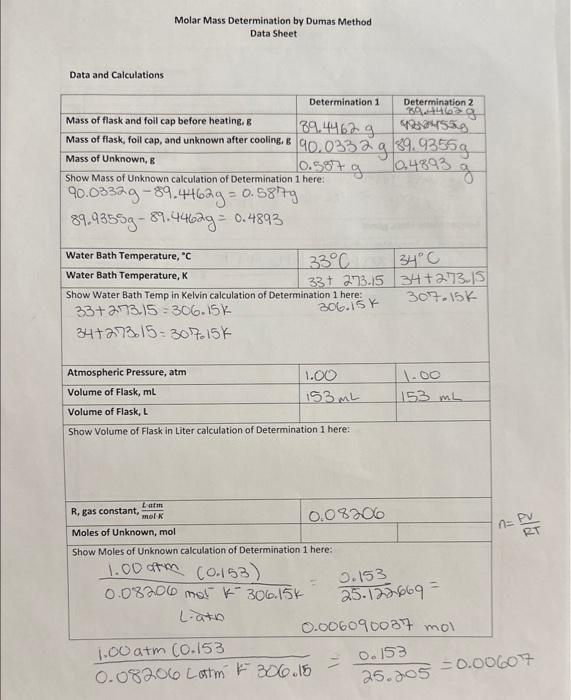


Molar Mass Determination bv Dumas Method 1_-at_- ha. Runnen A abkNat Questions 1. The unknown has a molar mass of 84.16g/mol. Use equation below, calculate the percent error of this experiment. 2. Why do we not have to determine the mass of the flask plus the original 5mL of unknown sample that was placed in it? 3. Suppose instead of using the actual volume the flask can hold, we used the volume printed on the flask. How would that affeet the number of moles calculated and the calculated molar mass? Briefly explain. 4. Suppose the unknown liquid did not completely vaporize. How would this affect the size of the molar mass that would be calculated? Briefly explain. 5. Suppose there were water droplets trapped under the foil eap after cooling. How would this affeet the calculated molar mass? Briefly explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


