Question: Here is the elemental composition (wt %) of a typical iron ore: Element Concentration7% Fe 59.2 Si 4.70 Al 0.908 V 0.193 Ca 0.095
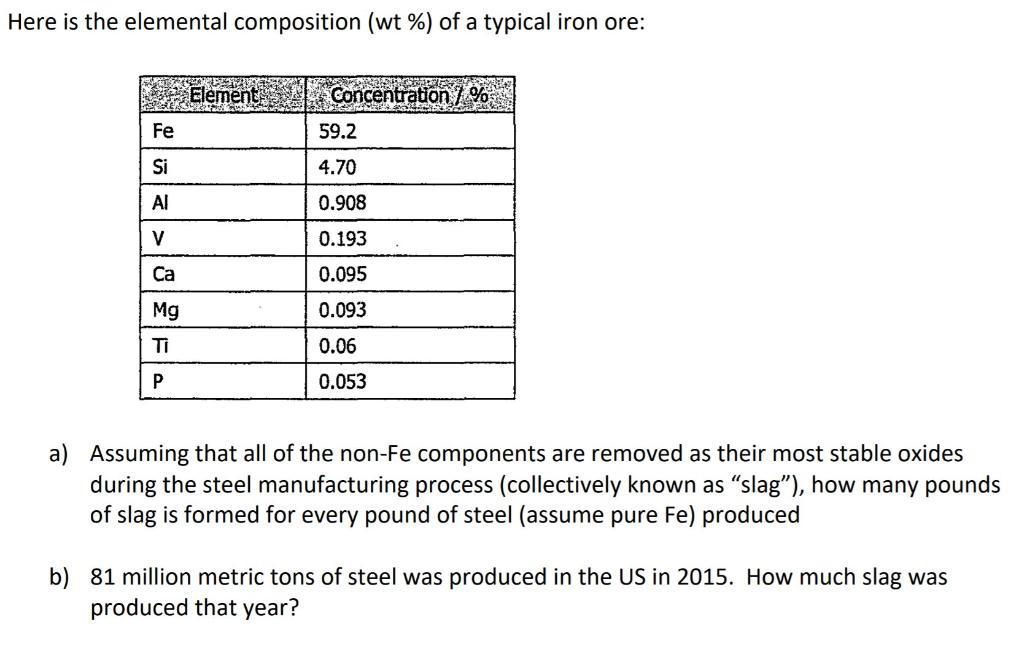
Here is the elemental composition (wt %) of a typical iron ore: Element Concentration7% Fe 59.2 Si 4.70 Al 0.908 V 0.193 Ca 0.095 Mg 0.093 Ti 0.06 0.053 a) Assuming that all of the non-Fe components are removed as their most stable oxides during the steel manufacturing process (collectively known as "slag"), how many pounds of slag is formed for every pound of steel (assume pure Fe) produced b) 81 million metric tons of steel was produced in the US in 2015. How much slag was produced that year?
Step by Step Solution
3.32 Rating (149 Votes )
There are 3 Steps involved in it
a Lets take the basis as 100 pounds of iron ore according to the data given Fe 592 ... View full answer

Get step-by-step solutions from verified subject matter experts


