Question: heres the full thing, can you do part 2 Culminating Lab Activity Analysis and Optimization of Trisodium Citrate Production The data provided in tables 1
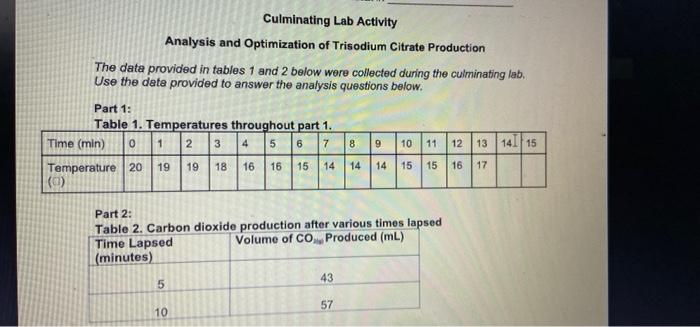

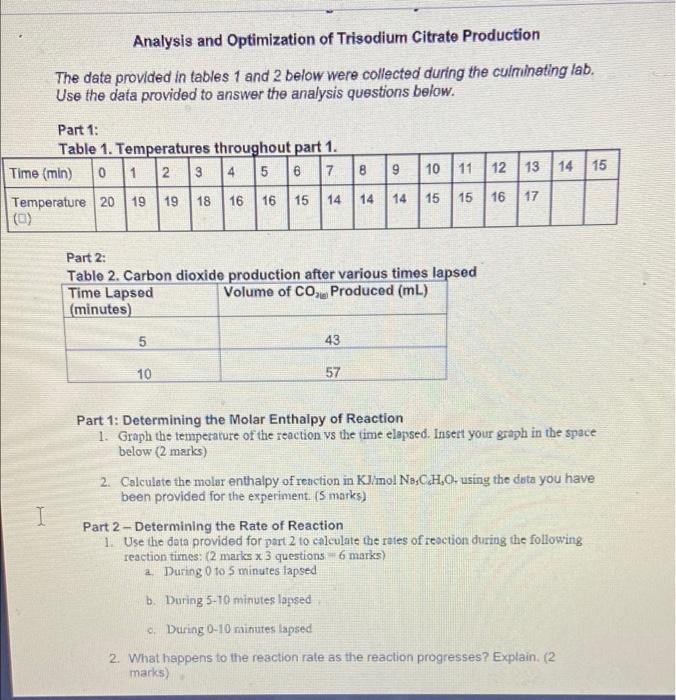
Culminating Lab Activity Analysis and Optimization of Trisodium Citrate Production The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Temperature 20 19 18 16 16 15 14 14 14 15 15 16 (a) 19 17 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO Produced (mL) (minutes) 5 43 57 10 Part 2 - Determining the Rate of Reaction 1. Use the data provided for part 2 to calculate the rates of reaction during the following reaction times: (2 marks x 3 questions - 6 marks) a. During 0 to 5 minutes lapsed b. During 5-10 minutes lapsed c. During 0-10 minutes lapsed 2. What happens to the reaction rate as the reaction progresses? Explain. (2 marks) Analysis and Optimization of Trisodium Citrate Production The data provided in tables 1 and 2 below were collected during the culminating lab. Use the data provided to answer the analysis questions below. 8 9 Part 1: Table 1. Temperatures throughout part 1. Time (min) 0 1 2 3 4 5 6 7 Temperature 20 19 19 18 16 16 15 14 (0) 10 11 12 13 14 | 15 14 14 15 15 16 17 Part 2: Table 2. Carbon dioxide production after various times lapsed Time Lapsed Volume of CO Produced (mL) (minutes) 5 43 10 57 Part 1: Determining the Molar Enthalpy of Reaction 1. Graph the temperature of the reaction vs the time elapsed. Insert your graph in the space below (2 marks) 2. Calculate the molar enthalpy of reaction in KJ/mol No;CHOusing the data you have been provided for the experiment. (5 marks) Part 2 - Determining the Rate of Reaction 1. Use the data provided for part 2 to calculate the roles of reaction during the following reaction times: (2 marks x 3 questions 6 marks) 2. During 0 to 5 minutes lapsed b. During 5-10 minutes lapsed . During 0-10 minutes lapsed - 2. What happens to the reaction rate as the reaction progresses? Explain. (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


