Question: Hess's Law: i only need help with the bottom two. explinations are greatly appreciated. Use Hess's Law the following problems From the following reactions: Pb
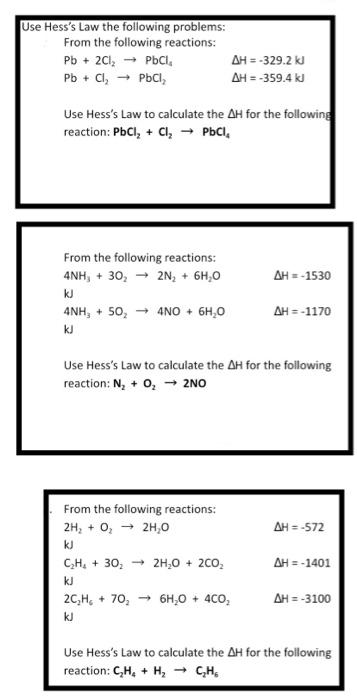
Use Hess's Law the following problems From the following reactions: Pb + 2Cl2 PCI AH-329.2 kJ Pb + Cl PCI AH = -359.4 kJ Use Hess's Law to calculate the AH for the following reaction: PbCl, + Cl, PbCl. AH-1530 From the following reactions: 4NH, + 30, 2N, + 6H,0 kJ 4NH, + 50, 4NO + 6H,0 kJ AH =-1170 Use Hess's Law to calculate the AH for the following reaction: N, + 0, 2NO AH = -572 From the following reactions: 2H2 + O, 2H,0 kJ CH. + 30,- 24,0 + 2CO, kJ 2CH. +70,- 64,0 + 4CO kJ AH = -1401 AH = -3100 Use Hess's Law to calculate the AH for the following reaction: CH + H2 CH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


