Question: Hey i need these done ASAP please.I need this to pass. C dZi Ebeal :a P30Module8Assignm pdf vloduieSAssigni X 9 7 100% + For this
Hey i need these done ASAP please.I need this to pass.
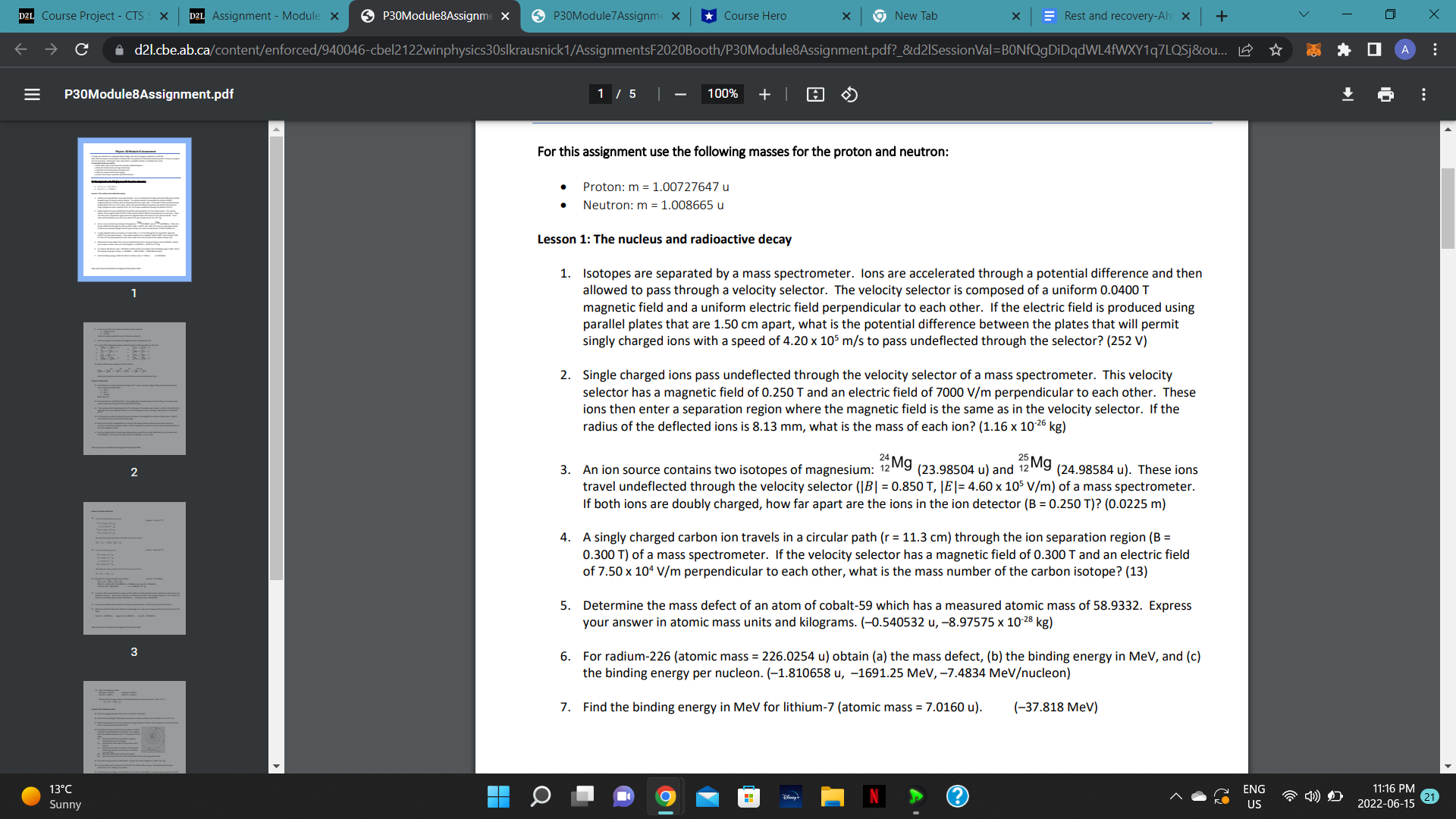
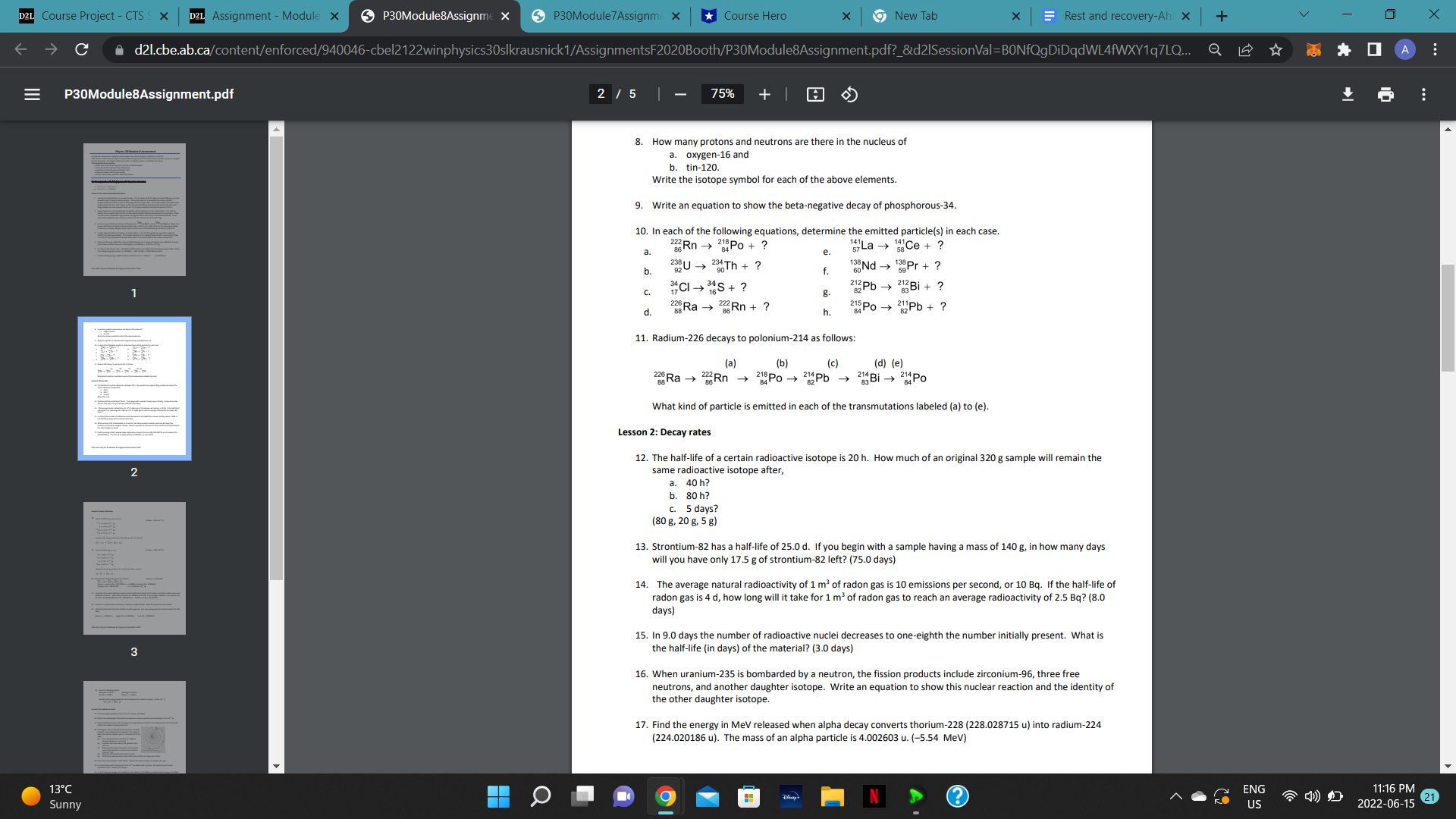
C dZi Ebeal :a P30Module8Assignm pdf vloduieSAssigni X 9 7 100% + For this assignment use the following masses forthe proton and neutron: Proton: m = 100727647 0 Neutron: rn : 1.008665 0 Lesson 1: The nucleus and radioactive decay 1. Isotopes are separated by a mass spectrometer. Ions are accelerated through a potential difference and then allowed to pass through a velocity selector. The velocity selector is composed of a uniform 0.0400 T magnetic field and a uniform electric field perpendicular to each other. If the electric field is produced using parallel plates that are 1.50 cm apart. what is the potential difference between the plates that will permit singly charged ions with a speed of 4.20 x 105 m/s to pass undeflected through the selector? (252 V) Single charged ions pass undeflected through the velocity selector of a mass spectrometer. This velocity selector has a magnetic eld of 0.250 T and an electric field of 7000 V/m perpendicular to each other. These ions then enter a separation region where the magnetic field is the same as in the velocity selector. If the radius of the deflected ions is 8.13 mm, what is the mass of each ion? [1.16 x 10'25 kg) 24 Mg 5 Mg An ion source contains two isotopes of magnesium: '2 [23.98504 u) and '2 (24.98584 u). These ions travel undeflected through the velocity selector [IBI = 0.850 T, |E|= 4.60 x 105 V/rn) of a mass spectrometer. If both ions are doubly charged, how far apart are the ions in the ion detector [B = 0.150 T)? [0.0125 m) A singly charged carbon ion travels in a circular path [r = 11.3 Cm) through the ion separation region (B : 0.300 T) of a mass spectrometer. If the velocity selector has a magnetic field of 0.300 T and an electric field of 7.50 x 10' V/m perpendicular to each other, what is the mass number of the carbon isotope? (13) Determine the mass defect of an atom of cobalt-59 which has a measured atomic mass of 58.9332. Express your answer in atomic mass units and kilogramS. (-0.540532 u, -B.97575 x 10.25 kg) For radium-226 [atomic mass = 226.0254 u) obtain (a) the mass defect. lb) the binding energy in MeV, and (c) the binding energy per nucleon. (1.810658 u, 1691.25 MeV, 4.4834 MeVucleon) Find the binding energy in MeV for lithium-7 (atomic mass = 7.0160 u). (-37.818 MeV) D2L Course Project - CTS S X D2L Assignment - Module x P30Module8Assignme X P30Module 7 Assignme X Course Hero x 9 New Tab X Rest and recovery-Ah x + V X C d21.cbe.ab.ca/content/enforced/940046-cbel2122winphysics30slkrausnick1/AssignmentsF2020Booth/P30Module8Assignment.pdf?_&d21SessionVal=BONfQgDiDqdWL4fWXY1q7LQ... Q 0 A E P30Module8Assignment.pdf 2 /5 | - 75% + 8. How many protons and neutrons are there in the nucleus of a. Oxygen-16 and b. tin-120 Write the isotope symbol for each of the above elements. 9. Write an equation to show the beta-negative decay of phosphorous-34. 10. In each of the following equations, determine the emitted particle(s) in each case. 2Rn - Po + ? "La - 58 Ce + ? a. e. b. Th + ? 138Nd -> Pr+ ? C. 7CI - #'S g. Pb - 8 Bi + ? d. 228Ra - 38 Rn + ? h 215Po -> Pb + ? 11. Radium-226 decays to polonium-214 as follows: (a) (b) (c) (d) (e) Ra - Rn - 214 Pb Bi - Po What kind of particle is emitted in each of the transmutations labeled (a) to (e). Lesson 2: Decay rates 12. The half-life of a certain radioactive isotope is 20 h. How much of an original 320 g sample will remain the 2 same radioactive isotope after, a. 40 h? b. 80 h? c. 5 days? (80 g, 20 g, 5 g) 13. Strontium-82 has a half-life of 25.0 d. If you begin with a sample having a mass of 140 g, in how many days will you have only 17.5 g of strontium-82 left? (75.0 days) 14. The average natural radioactivity of 1 m' of radon gas is 10 emissions per second, or 10 Bq. If the half-life of radon gas is 4 d, how long will it take for 1 m of radon gas to reach an average radioactivity of 2.5 Bq? (8.0 days) 15. In 9.0 days the number of radioactive nuclei decreases to one-eighth the number initially present. What is 3 the half-life (in days) of the material? (3.0 days) 16. When uranium-235 is bombarded by a neutron, the fission products include zirconium-96, three free neutrons, and another daughter isotope. Write an equation to show this nuclear reaction and the identity of the other daughter isotope. 17. Find the energy in MeV released when alpha decay converts thorium-228 (228.028715 u) into radium-224 (224.020186 u). The mass of an alpha particle is 4.002603 u. (-5.54 MeV) 13 C 9 N ? ENG 11:16 PM Sunny US 2022-06-15 21
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


