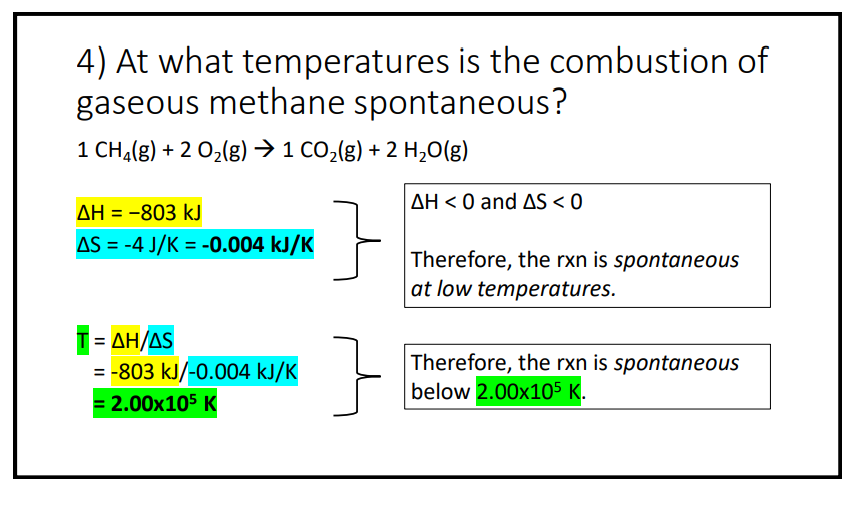
Question: hey, I was looking at these two questions. The question asked At what temperature is the combustion of gaseous methane spontaneous? From this, I am
hey, I was looking at these two questions. The question asked "At what temperature is the combustion of gaseous methane spontaneous?" From this, I am unsure how they got the heat reaction as -803kj and I am unsure how they got entropy (delta S) as -4J/k. Can you help me how they got these and provide an explanation.
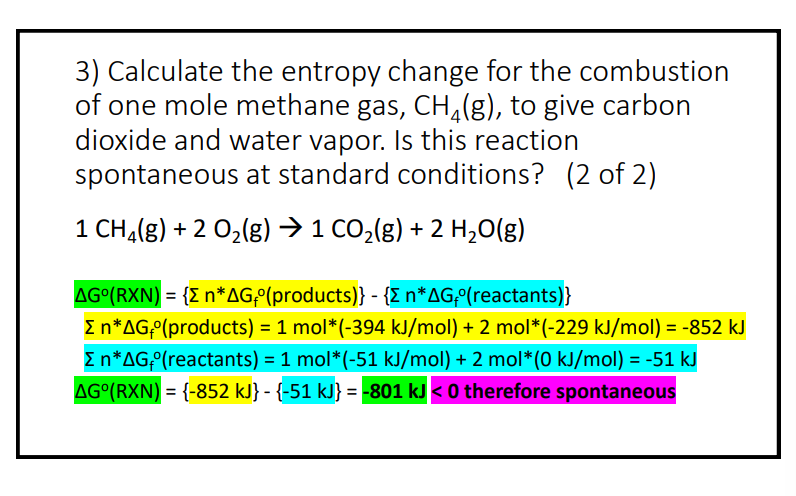
3) Calculate the entropy change for the combustion of one mole methane gas, CH4(g), to give carbon dioxide and water vapor. Is this reaction spontaneous at standard conditions? (2 of 2 ) 1CH4(g)+2O2(g)1CO2(g)+2H2O(g) G(RXN)={nGf( products )}{nGf( reactants )} nGf( products )=1mol(394kJ/mol)+2mol(229kJ/mol)=852kJ nGf( reactants )=1mol(51kJ/mol)+2mol(0kJ/mol)=51kJ G(RXN)={852kJ}{51kJ}=801kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


