Question: Hi! Could someone please show the calculation of (d), eventually incl. (a)(b)(c)? Thank you! A saturated-liquid mixture of 50mol% benzene (A) and toluene (B) is
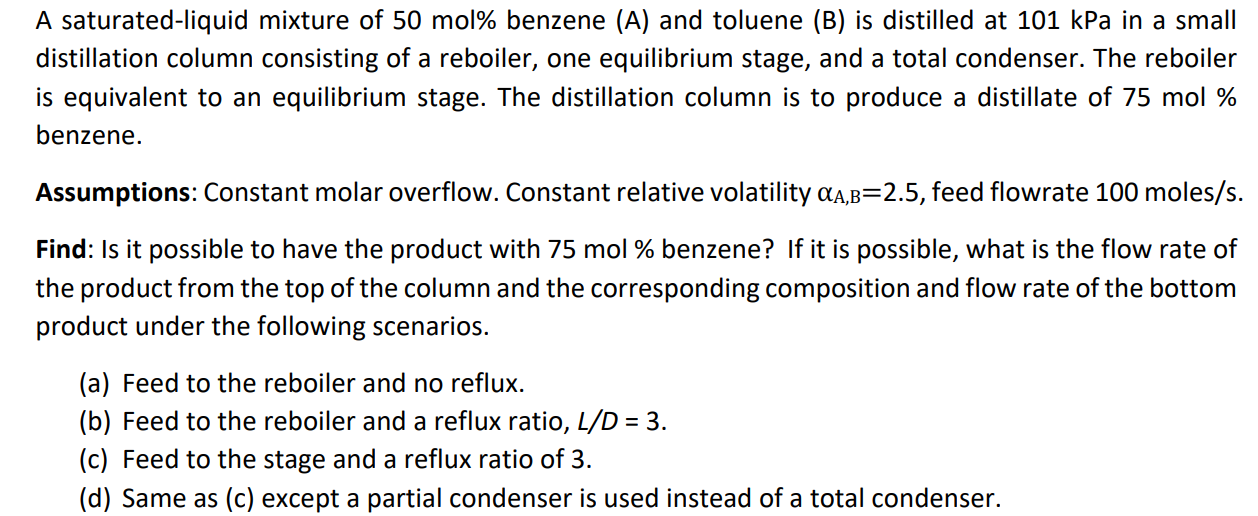
Hi! Could someone please show the calculation of (d), eventually incl. (a)(b)(c)?
Thank you!
A saturated-liquid mixture of 50mol% benzene (A) and toluene (B) is distilled at 101kPa in a small distillation column consisting of a reboiler, one equilibrium stage, and a total condenser. The reboiler is equivalent to an equilibrium stage. The distillation column is to produce a distillate of 75mol% benzene. Assumptions: Constant molar overflow. Constant relative volatility A,B=2.5, feed flowrate 100moles/s. Find: Is it possible to have the product with 75mol% benzene? If it is possible, what is the flow rate of the product from the top of the column and the corresponding composition and flow rate of the bottom product under the following scenarios. (a) Feed to the reboiler and no reflux. (b) Feed to the reboiler and a reflux ratio, L/D=3. (c) Feed to the stage and a reflux ratio of 3. (d) Same as (c) except a partial condenser is used instead of a total condenser
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


