Question: hi , i am looking for clear answer and step by step , please thanks 2. Ammonia is stripped from a dilute aqueous solution by
hi , i am looking for clear answer and step by step , please thanks
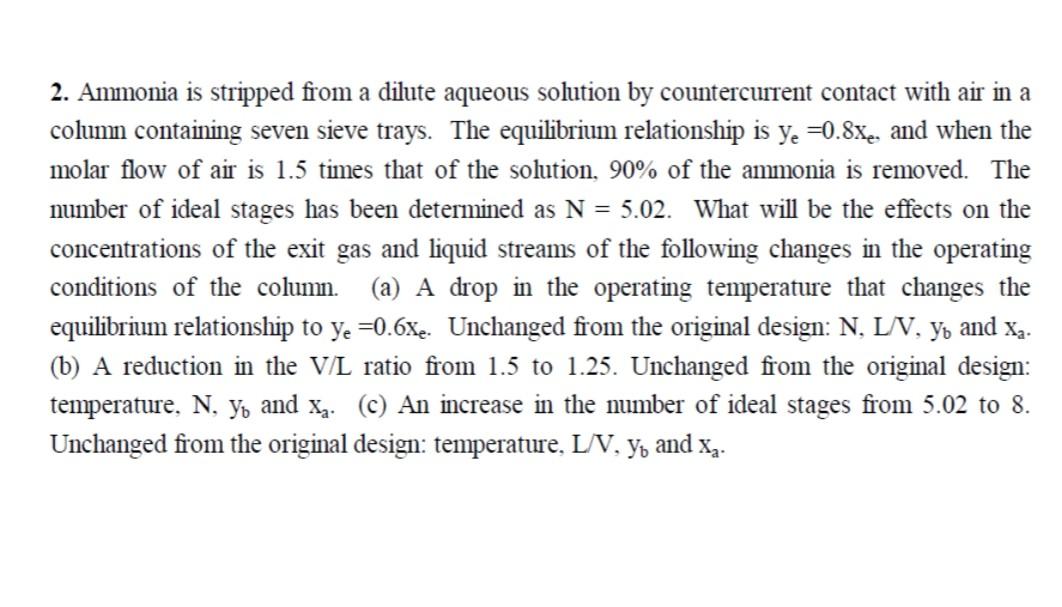
2. Ammonia is stripped from a dilute aqueous solution by countercurrent contact with air in a column containing seven sieve trays. The equilibrium relationship is ye=0.8xe, and when the molar flow of air is 1.5 times that of the solution, 90% of the ammonia is removed. The number of ideal stages has been determined as N=5.02. What will be the effects on the concentrations of the exit gas and liquid streams of the following changes in the operating conditions of the column. (a) A drop in the operating temperature that changes the equilibrium relationship to ye=0.6xe. Unchanged from the original design: N,L/V,yb and xa. (b) A reduction in the V/L ratio from 1.5 to 1.25. Unchanged from the original design: temperature, N,yb and xa. (c) An increase in the number of ideal stages from 5.02 to 8 . Unchanged from the original design: temperature, L/V,yb and xa
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


