Question: Hi! I need a bit oh help solving this multi-part question, thanks in advance! Question 6: Q6A: Cu2+ is dissolved in water to make a
Hi! I need a bit oh help solving this multi-part question, thanks in advance!
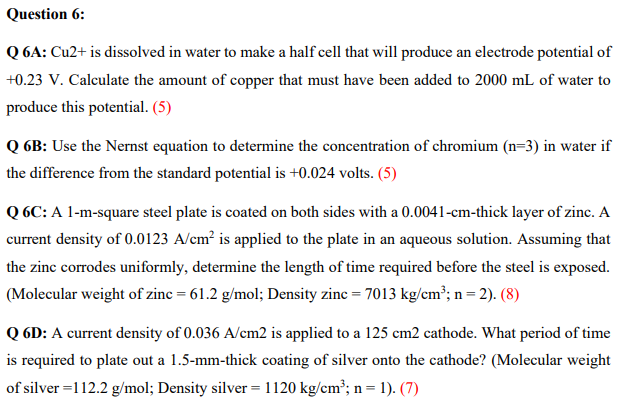
Question 6: Q6A: Cu2+ is dissolved in water to make a half cell that will produce an electrode potential of +0.23 V. Calculate the amount of copper that must have been added to 2000 mL of water to produce this potential. (5) Q6B: Use the Nernst equation to determine the concentration of chromium (n=3) in water if the difference from the standard potential is +0.024 volts. (5) Q6C: A 1-m-square steel plate is coated on both sides with a 0.0041-cm-thick layer of zinc. A current density of 0.0123 A/cm is applied to the plate in an aqueous solution. Assuming that the zinc corrodes uniformly, determine the length of time required before the steel is exposed. (Molecular weight of zinc = 61.2 g/mol; Density zinc = 7013 kg/cm; n=2). (8) Q6D: A current density of 0.036 A/cm2 is applied to a 125 cm2 cathode. What period of time is required to plate out a 1.5-mm-thick coating of silver onto the cathode? (Molecular weight of silver =112.2 g/mol; Density silver = 1120 kg/cm; n= n=1). (7)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


