Question: Hi! I need help with parts d, e, g please! And if possible, part i as well! Thank you so much A student prepares 50.0mL
Hi! I need help with parts d, e, g please! And if possible, part i as well! Thank you so much
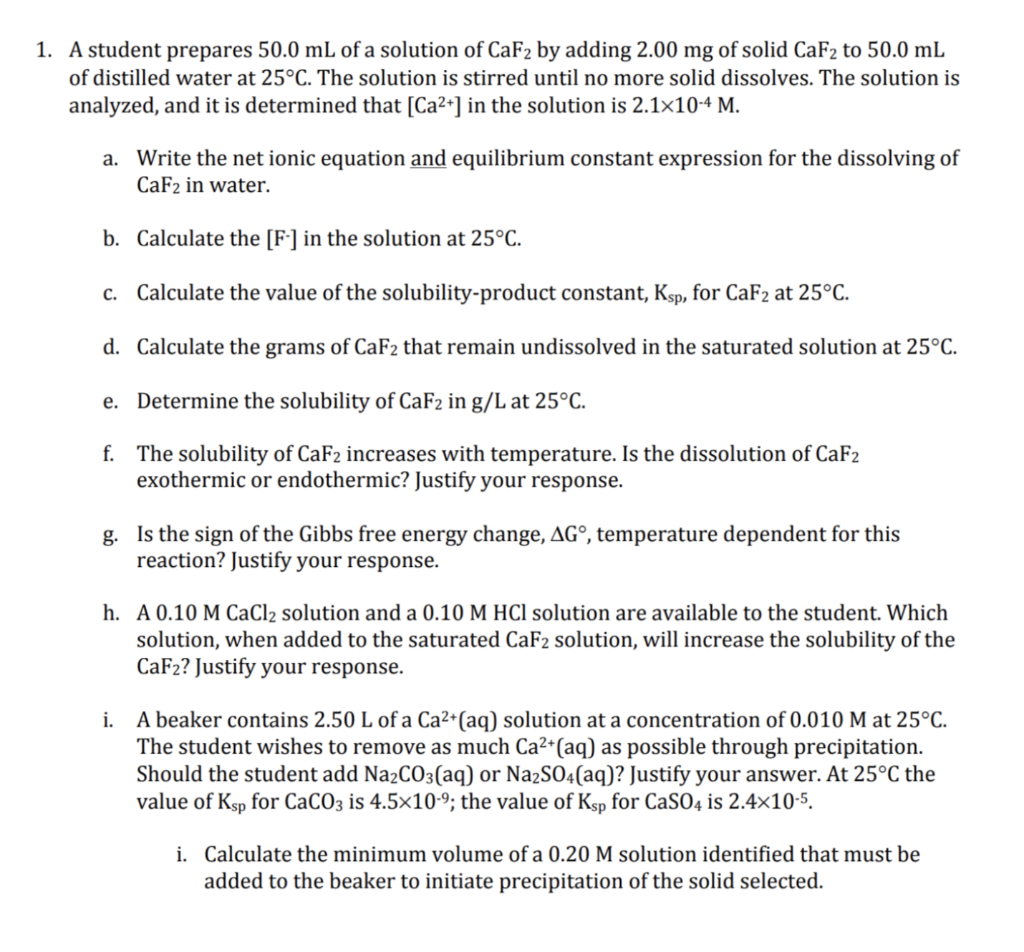
A student prepares 50.0mL of a solution of CaF2 by adding 2.00mg of solid CaF2 to 50.0mL of distilled water at 25C. The solution is stirred until no more solid dissolves. The solution is analyzed, and it is determined that [Ca2+] in the solution is 2.1104M. a. Write the net ionic equation and equilibrium constant expression for the dissolving of CaF2 in water. b. Calculate the [F - ] in the solution at 25C. c. Calculate the value of the solubility-product constant, Ksp, for CaF2 at 25C. d. Calculate the grams of CaF2 that remain undissolved in the saturated solution at 25C. e. Determine the solubility of CaF2 in g/ /L at 25C. f. The solubility of CaF2 increases with temperature. Is the dissolution of CaF2 exothermic or endothermic? Justify your response. g. Is the sign of the Gibbs free energy change, G, temperature dependent for this reaction? Justify your response. h. A 0.10MCaCl2 solution and a 0.10MHCl solution are available to the student. Which solution, when added to the saturated CaF2 solution, will increase the solubility of the CaF2 ? Justify your response. i. A beaker contains 2.50L of a Ca2+(aq) solution at a concentration of 0.010M at 25C. The student wishes to remove as much Ca2+(aq) as possible through precipitation. Should the student add Na2CO3(aq) or Na2SO4(aq) ? Justify your answer. At 25C the value of Ksp for CaCO3 is 4.5109; the value of Ksp for CaSO4 is 2.4105. i. Calculate the minimum volume of a 0.20M solution identified that must be added to the beaker to initiate precipitation of the solid selected
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


