Question: hi please help, follow the format below. Answer the PV DIAGRAM, HEAT SOLUTION, CHANGE IN ENERGY SOLUTION, WORK OF THE CYCLE SOLUTION. ANSWER ALSO THE
hi please help, follow the format below. Answer the PV DIAGRAM, HEAT SOLUTION, CHANGE IN ENERGY SOLUTION, WORK OF THE CYCLE SOLUTION. ANSWER ALSO THE QUESTION BELOW "HOW DOES INTERNAL ENERGY AND WORK AFFECT THE HEAT SYSTEM?"
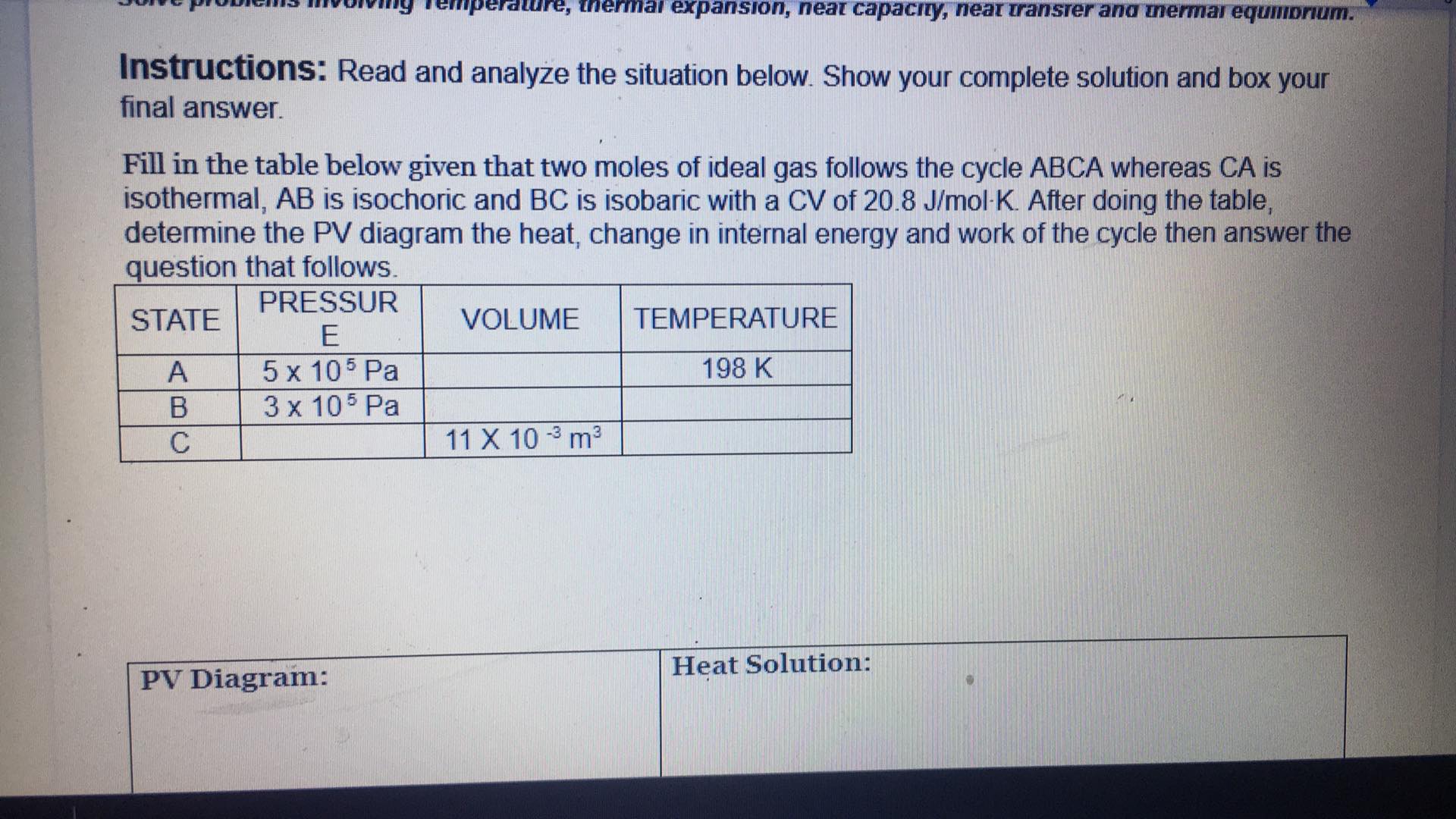
perature, thermal expansion, neat capacity, near Transfer and mermai equilibrium. Instructions: Read and analyze the situation below. Show your complete solution and box your final answer. Fill in the table below given that two moles of ideal gas follows the cycle ABCA whereas CA is isothermal, AB is isochoric and BC is isobaric with a CV of 20.8 J/mol-K. After doing the table, determine the PV diagram the heat, change in internal energy and work of the cycle then answer the question that follows. STATE PRESSUR E VOLUME TEMPERATURE A 5 x 105 Pa 198 K B 3 x 105 Pa C 11 X 10 -3 m3 PV Diagram: Heat Solution:3 5 6 7 8 Change in Internal Energy Solution: Work of the Cycle Solution: How does internal energy and work affect the heat of a system? Active Go to S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


