Question: Considering that Rxn 2 is taking place in the cathode while Rxn 1 is at the anode, determine the free energy change in kJ given
Considering that Rxn 2 is taking place in the cathode while Rxn 1 is at the anode, determine the free energy change in kJ given the following. Please show a complete solution. Note that this happens at a room temp.
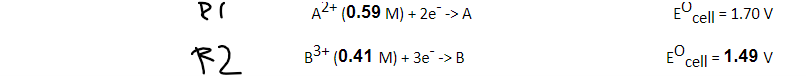
A2+ (0.59 M) + 2e -> A Ecell = 1.70 V 3+ (0.41 ) + 3 -> cell = 1.49 v %3D
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
636304c720354_237861.pdf
180 KBs PDF File
636304c720354_237861.docx
120 KBs Word File


