Question: hi please help me with this two question. tks 006 (part 1 of 2) 1.0 points It is necessary to add iodide ions to precipitate
hi please help me with this two question. tks 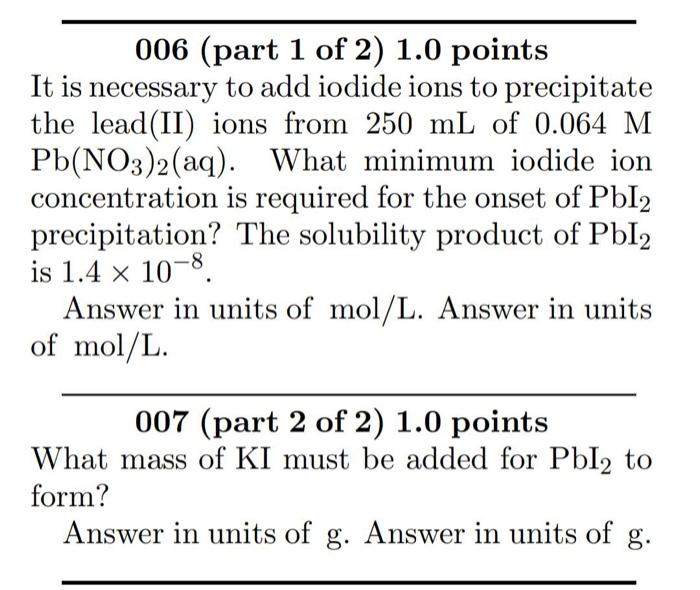

006 (part 1 of 2) 1.0 points It is necessary to add iodide ions to precipitate the lead(II) ions from 250mL of 0.064M Pb(NO3)2(aq). What minimum iodide ion concentration is required for the onset of PbI2 precipitation? The solubility product of PbI2 is 1.4108. Answer in units of mol/L. Answer in units of mol/L. 007(part2of2)1.0points What mass of KI must be added for PbI2 to form? Answer in units of g. Answer in units of g
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


