Question: Hi! The correct answer is D , and not B because my instructor says that due to balanced equation where 2y atoms disappear for every
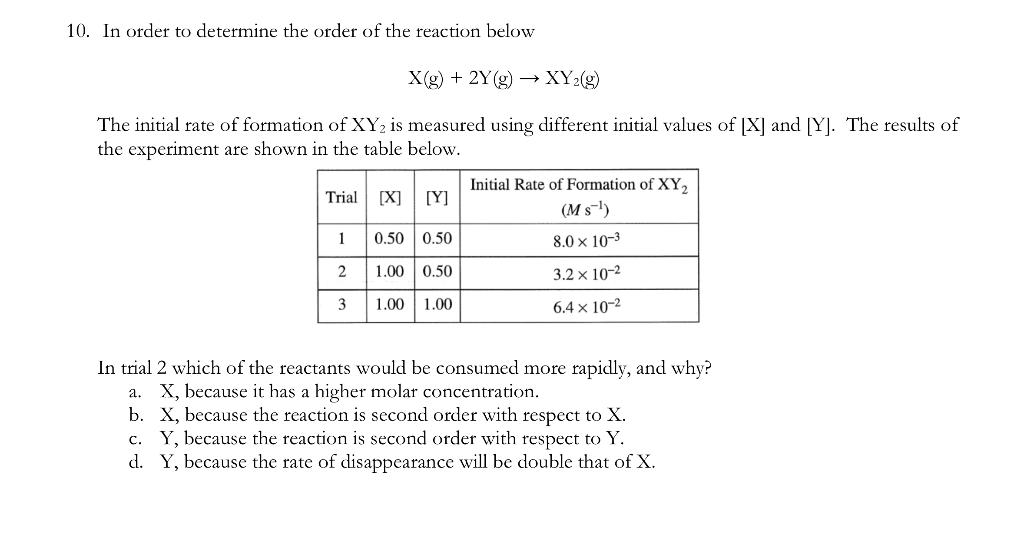
Hi! The correct answer is D, and not B because my instructor says that due to balanced equation where 2y atoms disappear for every x atom. However, I am confused why the answer is not B because reactants with second order are consumed much faster than reactants first order. PLEASE EXPLAIN WHY B IS NOT THE RIGHT ANSWER.
Thank you so much!
10. In order to determine the order of the reaction below X(g)+2Y(g)XY2(g) The initial rate of formation of XY2 is measured using different initial values of [X] and [Y]. The results of the experiment are shown in the table below. In trial 2 which of the reactants would be consumed more rapidly, and why? a. X, because it has a higher molar concentration. b. X, because the reaction is second order with respect to X. c. Y, because the reaction is second order with respect to Y. d. Y, because the rate of disappearance will be double that of X
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


