Question: Hi there i am having extreame difficulty doing pre lab homework can i please get help on the questions i have circled down below. please

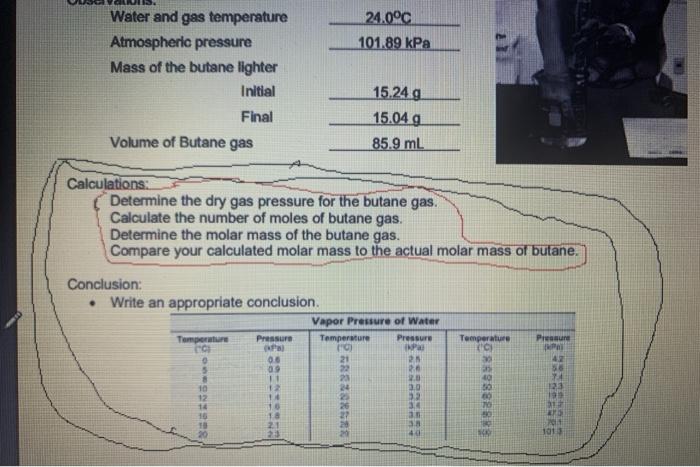
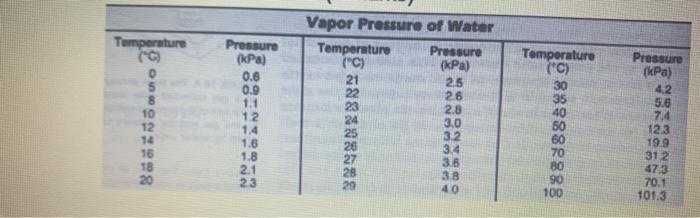
Determining the Molar Mass of Butane Purpose: To experimentally determine the molar mass of butane (CaH10). Materials: large beaker 100 ml graduated cylinder water . butane lighter electronic balance thermometer Procedure: 1. Fill the beaker % full with water. 2. Measure the temperature of the water. 3. Place the butane lighter under water. Remove and dry as thoroughly as possible. 4. Determine the mass of the butane lighter. 5. Fill the graduated cylinder to the top with water. Cover the top of the cylinder with a small piece of paper. Quickly invert and place the cylinder into the beaker taking care not to allow air into the cylinder. Remove the piece of paper. 6. Hold the butane lighter under the graduated cylinder. Depress the valve on the lighter to allow the butane gas to flow into the cylinder. 7. Allow approximately 50 mL of gas to enter the cylinder. Adjust the cylinder so the water level inside the cylinder is level with the water level outside the cylinder. Record the actual volume. 8. Thoroughly dry the butane lighter and then re-mass the lighter. Observations: Water and gas temperature 24.0C Atmospheric pressure 101.89 kPa Mass of the butane lighter Initial 15.24 Final 15.049 Volume of Butane gas 85.9 mb Calculations 1. Determine the dry gas pressure for the butane gas 2. Calculate the number of moles of butane gas 24.0C 101.89 kPa Water and gas temperature Atmospheric pressure Mass of the butane lighter Initial Final Volume of Butane gas 15.24 g 15.04 g 85.9 mL Calculations: Determine the dry gas pressure for the butane gas. Calculate the number of moles of butane gas. Determine the molar mass of the butane gas. Compare your calculated molar mass to the actual molar mass of butane. Conclusion: Write an appropriate conclusion Vapor Pressure of Water Temperature Pressure Temperature Pressure C Temperature { Press 06 09 11 21 22 5 IPad 25 2 2.0 30 12 42 58 24 10 12 888888888 10 30 18 21 40 Temperature (C) 0 Pressure (kPa) 0.6 0.9 1.1 12 1.4 1.6 1.8 2.1 23 8 10 12 Vapor Pressure of Water Temperature Pressure (C) (kPa) 21 2.5 2.6 23 2.8 24 3.0 25 3.2 26 3.4 27 36 38 29 4.0 Temperature ("C) 30 35 40 Pressure (kPa) 4.2 5.6 744 123 19.9 312 473 70.1 101.3 16 18 20 80 90 100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


