Question: Hint Check Answer [ Give Up? Resources KY 75.5% Assignment Score: Attempt 2 Isoclectric focusing can separate peptides based on their relative contents of acidic
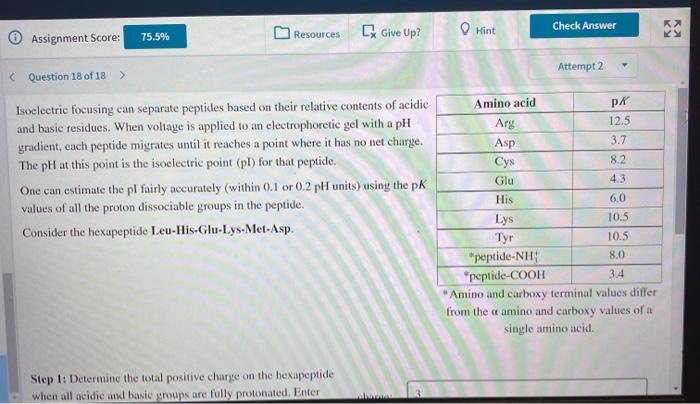
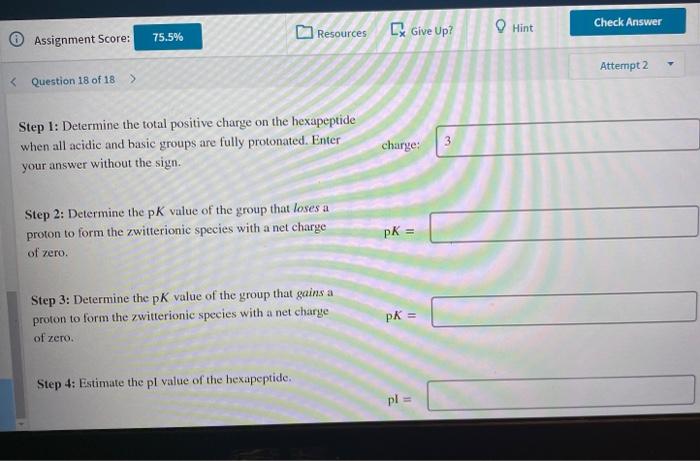
Hint Check Answer [ Give Up? Resources KY 75.5% Assignment Score: Attempt 2 Isoclectric focusing can separate peptides based on their relative contents of acidic and basic residues. When voltage is applied to an electrophoretic gel with a pH gradient, cach peptide migrates until it reaches a point where it has no net charge. The pH at this point is the isoelectric point (pl) for that peptide. One can estimate the pl fairly accurately (within 0.1 or 0.2 pH units) using the pk values of all the proton dissociable groups in the peptide. Consider the hexapeptide Leu-His-Glu-Lys-Met-Asp. Amino acid PK Ary 12.5 Asp 3.7 Cys 8.2 Glu 4.3 His 6.0 Lys Tyr 10.5 "peptide-NH: 8.0 *peptide-COOH 3.4 "Amino and carboxy terminal values differ from the camino and carboxy values of a single amino acid. 10.5 Step 1: Determine the total positive charge on the hexapeptide when all acidic and basic groups are fully protonated. Enter Check Answer Resources [ Give Up? Hint 75.5% Assignment Score: Attempt 2 Step 1: Determine the total positive charge on the hexapeptide when all acidic and basic groups are fully protonated. Enter your answer without the sign. charge: 3 Step 2: Determine the pK value of the group that loses a proton to form the zwitterionic species with a net charge of zero. PK = Step 3: Determine the pK value of the group that gains a proton to form the zwitterionic species with a net charge of zero. pk = Step 4: Estimate the pl value of the hexapeptide. pl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


