Question: In this problem, we want to estimate the ground state energy of the He atom using the variational principle. The He atom is a
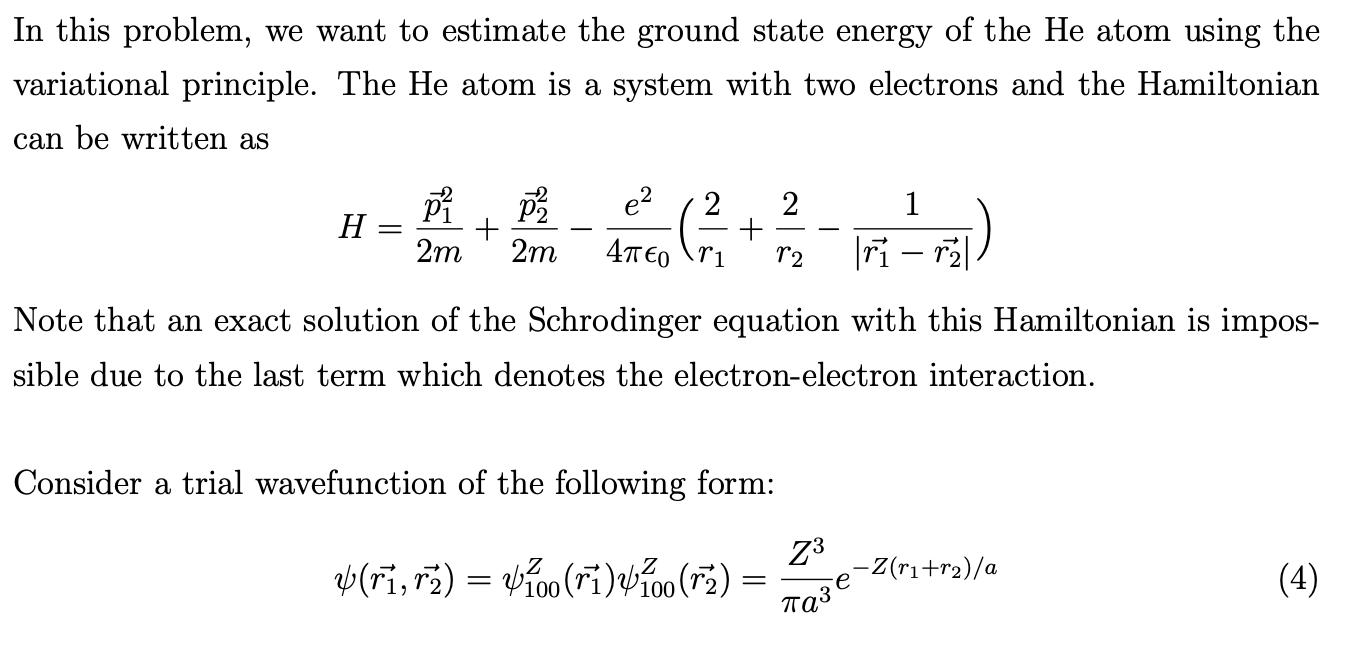


In this problem, we want to estimate the ground state energy of the He atom using the variational principle. The He atom is a system with two electrons and the Hamiltonian can be written as H = e 2 P P + 2m 2m 4EO + Consider a trial wavefunction of the following form: (ri, r) = 100 (ri) 100 (2) 2 = r2 Note that an exact solution of the Schrodinger equation with this Hamiltonian is impos- sible due to the last term which denotes the electron-electron interaction. Z 1 |ri - r| 3 -Z(r+r)/a (4) where Z is treated as a variational parameter, and 100 is the ground state wavefunction of a single-particle Hamiltonian for a hydrogenic atom with nuclear charge Ze. As we have learned in the lectures, the estimated ground state energy can be obtained by first obtaining the expectation value (H) using the trial wavefunction as a function of the variational parameter Z. For hydrogenic atoms, Let H = where p P2 + L2m 2m ]] 4700 (7) e Z 4 i H = -13.6eV e 2(4)a where a denotes the Bohr radius, E100 expectation value of 1/r when the wavefunction is given by It is therefore convenient to re-write the Hamiltonian as: 2m Eg = Z E100 1 (a/Z) (Vee) z Z e-2Z (r+r)/a |ri - r| = + Vee = Z a Ze 1 4or 3)] 72 + -Zr/a (H) = 2Z E100 + 2(Z - 2)- 40 r1 Z - 2 1 4 |ri - r| e 4EO 5Z 52 ()=-5Z100. 8 4 You may find the following integrals helpful: + Z (a) Show that, using the trial wavefunction, the expectation value of the Hamiltonian as a function of Z is = = e=221/0 and (r) = Z-2 -2Zr/ [r + (Vee) z () (a/Z) 1 + 12 |ri - r| dridr = [e-27/a[/=754 @]dn -drdr: dr dr (5) (6) (8) refers to the e-Zr/a (9) (10) (11) (12) 1 = -21 e-2Zr2/a dr2 is given by: 1 = 4 ( 1/1/ I2 e-2Zr/adr + Zr -2Zr/a 70 [1-(1+Z) e-22ri/a] Zr a + Se-22r2/0r2dr2) (13) Please refer to the Appendix for more details on how to obtain the expressions in Eq. 13. You should also use (r)r = (r)4rdr for f(r) that depends only on 7. It is useful to note that for Jn - 1.0 integration by parts gives the relation Jn = Jn-1. = re-ar dr, a>0 (b) Find the optimal value of Z, and calculate the ground state energy. What is the percentage error when compared with the experimental ground state energy of -79 eV? (c) Discuss whether you expect a similar variational approach to work well for the ground state of the Fe atom, and why the variational approach had worked so well for He. (Hint: consider if the trial wavefunction (Eq. 4) has taken into account what you have learnt about identical fermions.)
Step by Step Solution
3.45 Rating (174 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


