Question: How could spectroscopy be used to support that the below reaction occurred? Choose all that apply. H3O+ heat Choose one or more: UV-vis will not
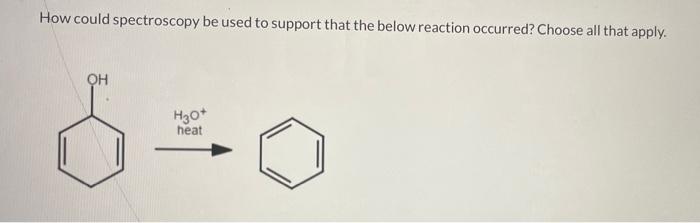

How could spectroscopy be used to support that the below reaction occurred? Choose all that apply. H3O+ heat Choose one or more: UV-vis will not be useful as no change in max would occur. Use IR; the product has a sharp narrow peak at 3100cm1, but the starting material does not. IR will not be useful as no detectable change in the spectrum will occur. Use IR; the product has a strong broad peak at 32003400cm1, but the starting material does not. Use UV-vis; the product would show an absorbance at a shorter max from that of the starting material. Use IR; the starting material has a sharp narrow peak at 3100cm1, but the product does not. Use UV-vis; the product would show an absorbance at a longer max from that of the starting material. Use IR; the starting material has a strong broad peak at 32003400cm1, but the product does not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


