Question: How do I do the whole question 2? QUESTION 2 (15 marks) 2.1 An oxygen-demanding waste containing organic matter (mainly formic acid, HCOOH) is continuously
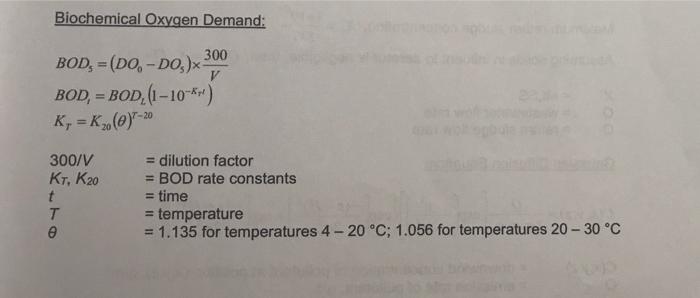
QUESTION 2 (15 marks) 2.1 An oxygen-demanding waste containing organic matter (mainly formic acid, HCOOH) is continuously discharged in a clean river stream. Upstream of the outfall, the river has a flow rate of 0.3 m/s and contains 7.0 mg/L of dissolved oxygen (DO). (a) Explain how the DO changes as a function of the downstream distance. (3 marks) (b) Suppose the formic acid discharge rate is 280 kg/day, estimate the DO at some distance downstream of the outfall to account for the complete organic waste decomposition by microbes. (4 marks) 2.2 A wastewater sample with a DO content of 1.4 mg/L is diluted with deionized water with a DO content of 8.8 mg/L in a 300 mL BOD bottle. The DO content of the diluted sample during a BOD test at 25C is 7.8 mg/L initially, 3.2 mg/L after 7 days, and 1.5 mg/L after an indefinitely long period of time. (a) Determine the volume of the undiluted wastewater sample used. (1 mark) (b) Determine the BOD5 of the undiluted wastewater sample if a test is conducted at 20 C. (7 marks) Biochemical Oxygen Demand: 300 BOD, E (DO, -DO) V BOD, = BOD, (1-10-*) K, K. (@)-20 20 300/V K1, K20 = dilution factor = BOD rate constants = time = temperature = 1.135 for temperatures 4-20 C; 1.056 for temperatures 20 - 30 C T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


