Question: how do i do this? (include steps) 3. A.) Give the balanced equation for the reaction generating oxygen gas in this experiment: 2 KClOsci) zlecless
how do i do this? (include steps)
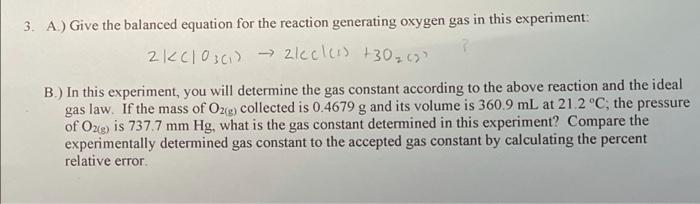
3. A.) Give the balanced equation for the reaction generating oxygen gas in this experiment: 2 KClOsci) zlecless +30 2620 B.) In this experiment, you will determine the gas constant according to the above reaction and the ideal gas law. If the mass of O2(g) collected is 0.4679 g and its volume is 360.9 mL at 21.2C, the pressure of O2(g) is 737.7 mm Hg, what is the gas constant determined in this experiment? Compare the experimentally determined gas constant to the accepted gas constant by calculating the percent relative error
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


