Question: How do i do this problem in excel? 1. (50 pts) The Langmuir adsorption isotherm can be used to model the adsorption of Co(II) ions
How do i do this problem in excel?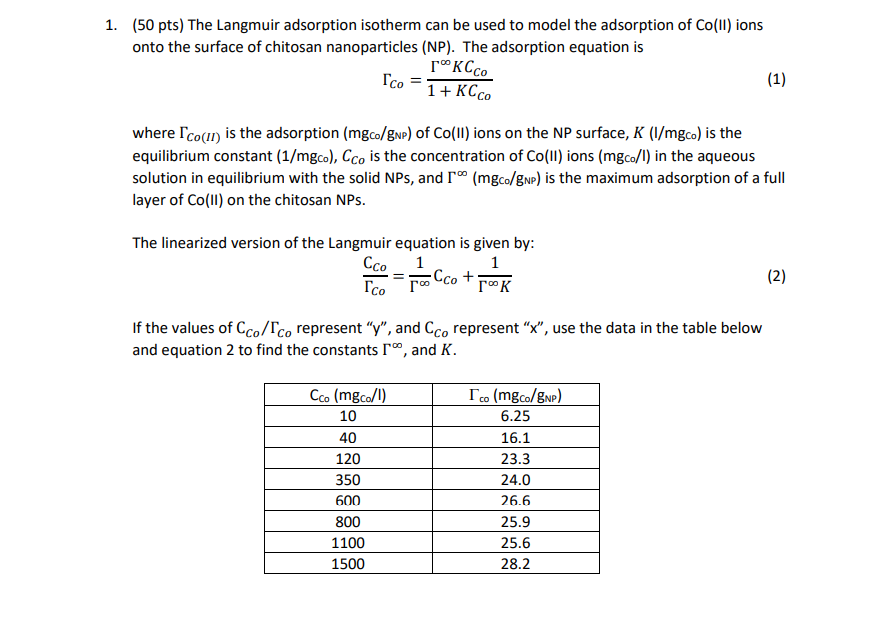
1. (50 pts) The Langmuir adsorption isotherm can be used to model the adsorption of Co(II) ions onto the surface of chitosan nanoparticles (NP). The adsorption equation is Co=1+KCCoKCCo where Co(II) is the adsorption ( mgC0/gNP) of Co(II) ions on the NP surface, K(I/mgC0) is the equilibrium constant (1/mgc0),CCo is the concentration of Co(II) ions (mgC0/l) in the aqueous solution in equilibrium with the solid NPs, and (mgC0/gNP) is the maximum adsorption of a full layer of Co (II) on the chitosan NPs. The linearized version of the Langmuir equation is given by: CoCCo=1CCo+K1 If the values of CCo/Co represent " y ", and CCo represent " x ", use the data in the table below and equation 2 to find the constants , and K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


