Question: how do i find the initial subscripts from molar ratio? reported to TWO sig figs copper x subscript and oxygen y subscript CuxOy below is

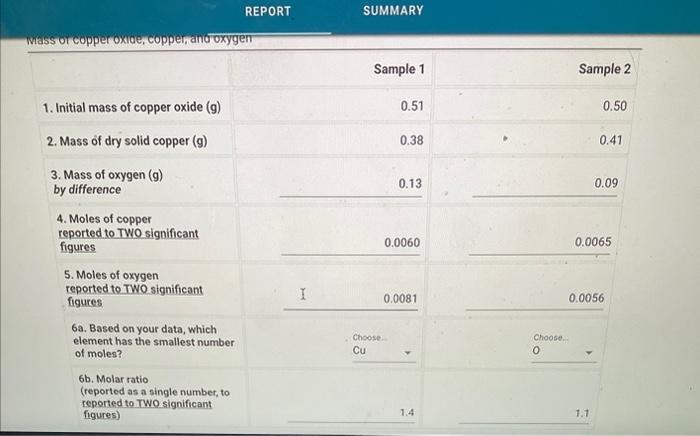

Determining the initial subscripts x and y in the CuxOy product from the molar ratio REPORT SUMMARY Whass or copper oxide, copper, anc oxygen Sample 1 Sample 2 1. Initial mass of copper oxide (g) 2. Mass of dry solid copper (g) 3. Mass of oxygen (g) by difference 0.13 0.09 4. Moles of copper reported to TWO significant figures 0.0060 0.0065 5. Moles of oxygen reported to TWO significant figures. I 0.0081 0.0056 6a. Based on your data, which element has the smallest number of moles? 6b. Molar ratio (reported as a single number, to reported to Two significant figures) 1.4 1.1 4ab3 F moles of C7=63.55g/molcu0.38gCu[0.005979544]5 0.0060 3 moles of cu 2=63.55g/molcu0.41gcu=[0.006451613]0.0065 $ moles of O1 =16.00gmol0.13g=[0.00812]0.0081 \# moles of 02=14.00gmolc0.09n=[0.005625] 0.006 =0/000.0059795440.008125=[1.358799264]1.45RBatio2=0.0064516130.005625=[0.871874987][0.87
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


