Question: How do I solve 2.2 to get the answer provided? help please 2.2 A wastewater sample has an ultimate BOD (BODL) of 380 mg/L and

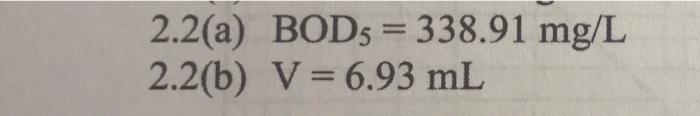
2.2 A wastewater sample has an ultimate BOD (BODL) of 380 mg/L and dissolved oxygen (DO) content of 1.5 mg/L. The sample is tested for BODs using 300-ml BOD bottles under standard testing conditions. (a) Given that the BOD rate constant at 18 C is 0.15 day, determine the BODs of the wastewater sample. (4 marks) (b) Deionized water with a DO content of 9.0 mg/L is used to dilute the wastewater sample for BODs measurement. Calculate the maximum volume of sample that can be used so that the DO of the diluted sample does not drop below 1.0 mg/L at the end of the five-day measurement period. (8 marks) 2.2(a) BOD5 = 338.91 mg/L 2.2(b) V = 6.93 mL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


