Question: How do I solve this? Consider two identical insulated sealed volumes, each with V : 10m3. One of the volumes is initially lled with an
How do I solve this?
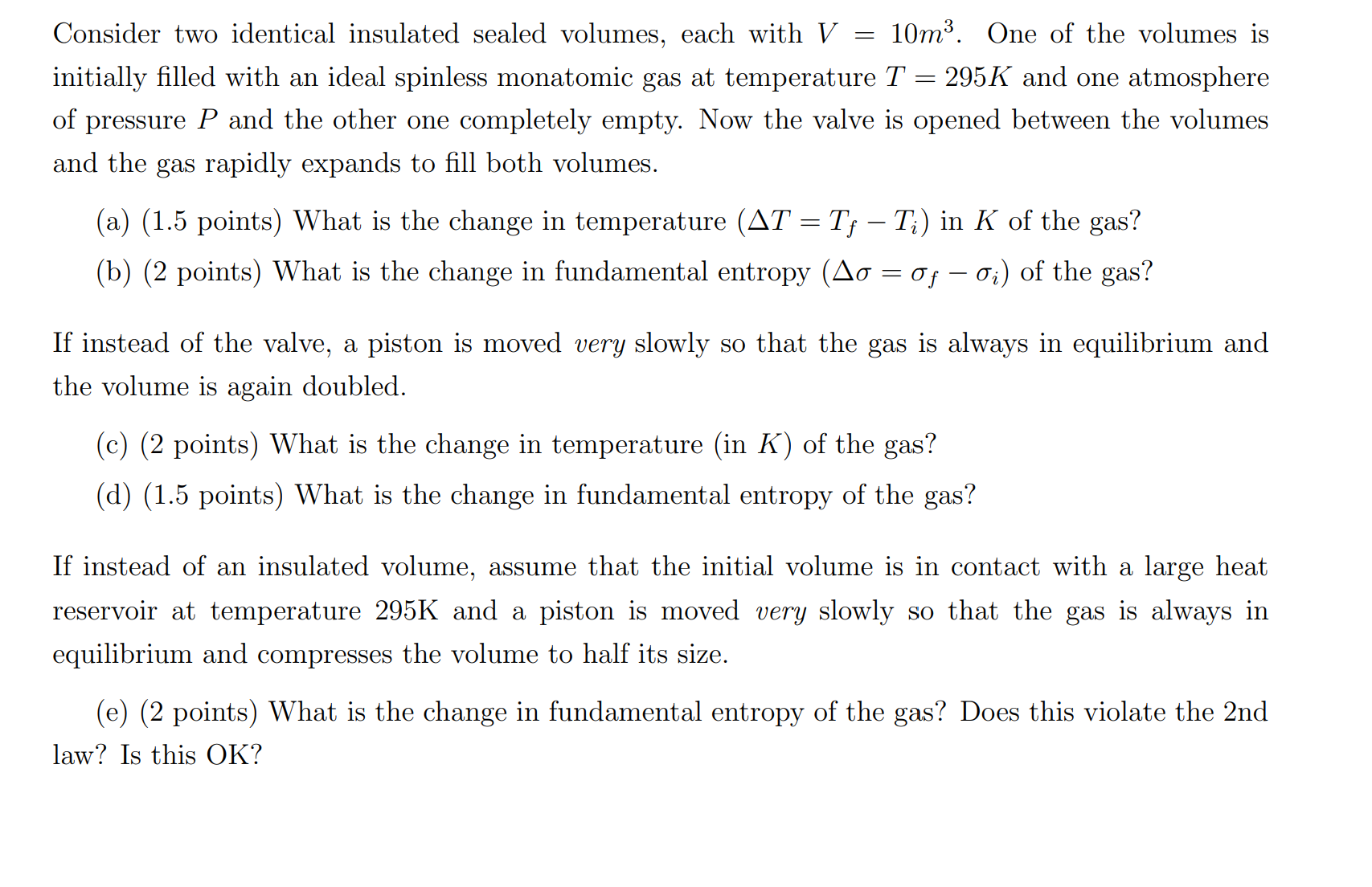
Consider two identical insulated sealed volumes, each with V : 10m3. One of the volumes is initially lled with an ideal spinless monatomic gas at temperature T : 295K and one atmosphere of pressure P and the other one completely empty. Now the valve is opened between the volumes and the gas rapidly expands to ll both volumes. (a) (1.5 points) What is the change in temperature (AT : Tf TJ in K of the gas? (b) (2 points) What is the change in fundamental entropy (A0 : of 07;) of the gas? If instead of the valve, a piston is moved very slowly so that the gas is always in equilibrium and the volume is again doubled. (c) (2 points) What is the change in temperature (in K) of the gas? (d) (1.5 points) What is the change in fundamental entropy of the gas? If instead of an insulated volume, assume that the initial volume is in contact with a large heat reservoir at temperature 295K and a piston is moved very slowly so that the gas is always in equilibrium and compresses the volume to half its size. (e) (2 points) What is the change in fundamental entropy of the gas? Does this violate the 2nd law? Is this OK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


