Question: how do u solve a and b please weite down the stepps clearly i havent had chem in years! 6. Perform the following operation; put
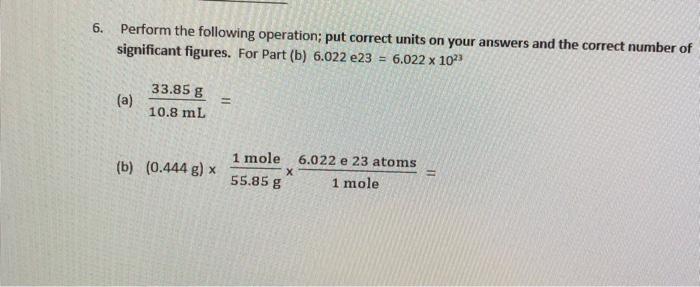
6. Perform the following operation; put correct units on your answers and the correct number of significant figures. For Part(b) 6.022 e23 = 6.022 x 10 (a) 33.85 g 10.8 ml (b) (0.444 g) x 1 mole 6.022 e 23 atoms 1 mole 55.85 g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


