Question: how do you answer questions 3 and 4 3. Write the rate expression. In the form rate = k [H2O21[1]. (you still don't know k)

![expression. In the form rate = k [H2O21"[1]". (you still don't know](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8d21d7b29f_74966f8d21d2399b.jpg)
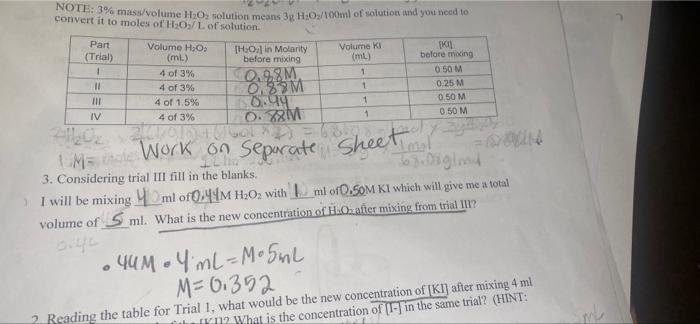
3. Write the rate expression. In the form rate = k [H2O21"[1]". (you still don't know k) 4. Calculate k for each trial and fill in on the data analysis table. Advanced Chemistry with Vernier we need to it to Min for moll's). You can do so using the ideal low as follows show work) M-WRT 14 .6 (R-8.314 L. Pa/mol K) -3 For the after mixing concentrations consider the MV - M:Vacquation. Where Vi is the initial volume used and V, is the new combined volume. Part Rate constant 1 95x105 3.5*105 2 Hubuns Il Initial rate [H0] (mol/L-5) after mising after mixing to.plo.c00095 6.70M O.IM D.Jan 0.000035 0.704M 6.05M 9.0010-a 0.000024 0.352 M 488108 ODOOZTS 0.700M OM 5.55*107 !! O.IM 2.7 x 104 IV Show your work for filling in the above table. MEPISE SEA . 3 'M= 0.0096 Separate Sheet [H0] in Molarity before mixing Volume ki (mt) 1 Part (Trial) 1 HI HII IV NOTE: 3% masa/volume Honolution means 38 H.O/100ml of solution and you need to convert it to moles of H.O/L of solution KI before ming 0.98M 0:50 M 0.89M 0.25 M 0.99 0.50 M 0.1 0.50 M HUGOL NIP ME SON 3. Considering trial III fill in the blanks Joiglo I will be mixing 4 ml of 0.44M H:02 with |_mi ofO.SOM KI which will give me a total volume of 5 ml. What is the new concentration of H.O after mixing from trial ? Volume H.O. (ml) 4 of 3% 4 of 3% 4 of 1.5% 4 of 3% 1 1 1 1. Maine Work on separate Sheet 4UMYmL=Mo5nL M = 0.352 2 Reading the table for Trial 1, what would be the new concentration of [KI) after mixing 4 ml IKT What is the concentration of 1 in the same trial? (HINT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


