Question: - How do you build a comprehensive risk management system based on FDA? -9.From 2005 through 2009, FDA received approximately 56,000 reports of adverse events
- How do you build a comprehensive risk management system based on FDA?
-9.From 2005 through 2009, FDA received approximately 56,000 reports of adverse events associated with infusion pumps, including numerous injuries and deaths." What are the current numbers 2022? what numbers are in KSA? check sfda or any other reference.
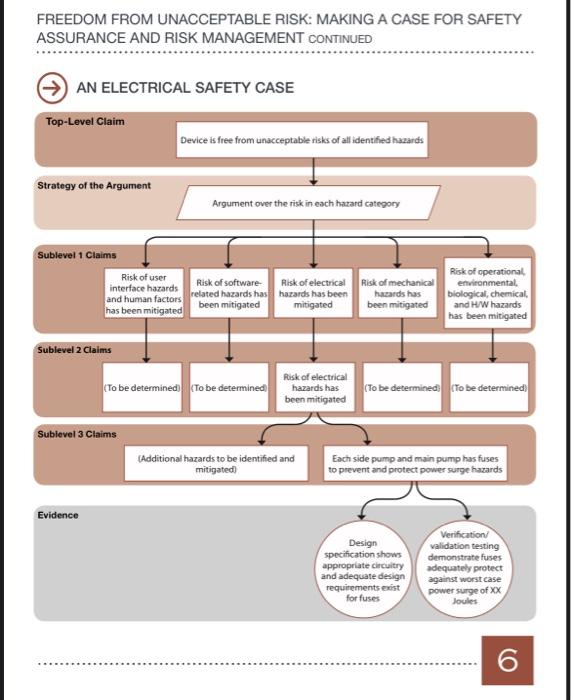
An infusion pump company considers risk assessment and mitigation induding numerous injuries and deaths. During this time period, MAKING A CASE FOR SAFETY ASSURANCE firms conducted 87 infusion pump AND RISK MANAGEMENT recalls to identify and resolve safety concerns. We want to know why some of our competitors had their THISFICTIONALIZED CASE STUDY IS THE SECOND IN AN EDUCATOONAL. products recalled so we can avoid SERIES PUOUSHED EY THE US. FOOD ASD DFUG ADMivistranon. the same mistakes." (See box, next Heary Neyhard looked across the simple user features, increasing page, for the problems reported long polished conference table and patient compliance and ultimately most frequently.) liked what he saw. He was meeting improving public health. Although with Sten Laws and Rush Mooney, the initial design focused on Neyhard rubbed his temples, 7 the first two employees of his adult patients, Neyhard, a retired knew there were recalls, but those fledgling medical device company. pediatrician, planned to adapt the numbers are striking." He had recruited them fresh out of device for pediatric diabetic care. He grad school, and their intelligence had every confidence in his product. Laws, the company's director and dedication to building the His chief concern now was getting of business development and company were impressive. Today the regulatory approval he would regulatory affairs, nodded. "I know, they were presenting to Neyhard their research on U.S. Food and for FDA, also known as a Neyhard had invented a cost- S10(k). Neyhard had started down and we want to make sure that effective infusion pump that could this road before with other devices doesn't happen to us. But FDA wants deliver insulin at preset rates with he had developed, but his lack of the same thing. The agency wants regulatory knowledge had been too design deficiencies to be identified much to overcome. This time, he and corrected before they lead to was determined to be prepared. safety problems. FDA is moving to require manufacturers of infusion Regulatory Background pumps to include additional design and enginecring information as Mooney, the company's director of part of their premarket submission, product development, spoke first. detailing the steps taken to mitigate 'In recent years, FDA's Center for risks at each stage of the device's life Devices and Radiological Health. cycle: design, manufacture, servicing or CDRH, has recognized that and maintenance, and use. And infusion pumps have significant FDA recommends design validation safety issues. From 2005 through testing specific to the setting where 2009, FDA received approximately 56,000 reports of adverse events FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTINUED FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTINUED FDA's expectations. We've done weighs risks and benefits. In fact. confident. We need to present the risk mitigation throughout the the standard refers to safety as the evidence point by point in an life cycle so far, we've done the " 'rreedom from unacceptable risk" organized way that adds up. The validation testing, and we know But the standard does not explicitly safety assurance case is a map that about FDA's risk management require an assurance case." can get us where we want to go." standard. The standard requires _ The activities required by FDA's risk AN INFUSION PUMP DESIGN FLAW: PUMP FIRES One of the most serious issues with intusion pumps is reports of sparing and farres when pump modules are attached to a running unit One compaty attrbuted this to poor user mantenance, even atter ample evidence contradicled this theory. The company made design changes that reduced but did not eliminate the problem, because the changes did not address the underlyng causes. FDA andysis of laled connectors revealed design deficiencies not addressed by the company. Below are photomicrograghs of taled connectors taken in the CDRH mechanical FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTINUED THE EXPERTS RESPOND riskestimation,riskcontrol,evidenceofimplementation,andrisk/benefitanalysis-makeupacriticalpartofthesafetyassurancecase.Infact,theassurancecaseisacomprehensive,systematicrisk"lmallaboutefficiency,"answeredLaws."Butinthiscase,itsactuallymoreefficienttodomore.Andreally,howmuchmoreisit?Likeyousaid,werealreadydoingexemplaryriskmanagement.ThisApplicationofRisk.AssessmentandMitigationLawswonherargumentwithMooney;shewasabletoconvincehimthattheyshoulddothesafety management report. By conducting is just a framework for providing case. A week after their last meeting the risk management staadard the information. By doing the with Neyhard, the three reconvened activities within the structured assurance case, we will meet FDA's in the conference room. framework of an assurance case, expectations and increase our Laws argued, compantes can assure likelihood of regulatory approval. Mooney clicked on the first slide in that there are no gaps. And most importantly, it will help his presentation. "Dr. Neyhard, our ensure that our pump is safe and risk mitigation strategy covers nine "I thought it would be more efficient decrease the chance of adverse areas of safety concern: electrical, and cost effective to avoid doing events and recalls." mechanical, software, hardware, an assurance case," said Mooney. "I thought that's what you would want," FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTINUED FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTINUED AN ELECTRICAL SAFETY CASE Top-Level Claim Device is free from unacceptable risks of all identified hazards Strategy of the Argument Argument over the risk in each harard category Sublevel 1 Claims Sublevel 2 Claims \begin{tabular}{c|c|c|c|c|} Risk of user interface hazards and human factors has been mitigated & Risk of softwarerelated hazards has been mitigated & Risk of electrical hazards has been mitigated & Risk of mechanical hazards has been mitigated \\ \hline \end{tabular} Risk of operational, efvironmental, biological, chemical, and HWW hazards has been mitigated Sublevel 3 Claims Evidence (Additional hazards to be identified and Each side pump and main pump has fuses mitigated) to prevent and protect power surge hazards FREEDOM FROM UNACCEPTABLE RISK: MAKING A CASE FOR SAFETY ASSURANCE AND RISK MANAGEMENT CONTNNUED GLOSSARY Approval: Approval of a medical device must be Guidance Documents: Documents prepared for FDA obtained from the FDA by demonstrating that the _ staff, applicants/sponsors, and the public that describe device is reasonably safe and effective, and that the the agency's interpretation of or policy on a regulatory benefits outweigh the risks for the intended patient_ issue. They do not create or confer any rights for or population before it can be put into commerce. on any person and do not operate to bind FDA or the public. An altermative approach may be used if such Eriticai Control Pointi A point, step, or procedure in a _ approach satisfies the requirements of the applicable process at which control can be applied, and a hazard statute, regulations, or both. Draft guidance documents can as a result be prevented, eliminated, or reduced to are for the public to comment on and suggest changes acceptable levels. for, but are not for implementation. (See 21 CFR Part Critical Limit: A maximum or minimum value to which 10.115 [b]. [d], and [8]] a biological, chemical, or physical parameter must be controlled at a critical control point to prevent. eliminate, or reduce to an acceptable level the Hazant: Potential source of harm. occurrence of the identifled safety hazard. Hazardoos Situation: Circumstance in which people, property, or the environment is exposed to one or Effectiveness: There is reasonable assurance that a device is effective when it can be determined, based Intended Use/fndication For Use: "Intended use" upon valid scientific evidence, that in a significant means the general purpose of a device, or what the more hamard(s). portion of the target population, the use of the device device does, and encompasses the indications for for its intended uses and conditions of use will provide use. "Indications for use" describes the disease or clinically significant results, 221 CFR Part 860.7) condition the device will diagnose, treat, prevent, cure, or mitigate, including a description of the patient population for which the device is intended