Question: How do you go about this question? I'm not sure how I am supposed to know what the reactants are? Explain please. N2O4(s)=35.0N2H4(l)=50.63H2O(l)=285.83HNO3(g)=134.3N2O4(l)O3(g)=19.56=+142.3 Write out
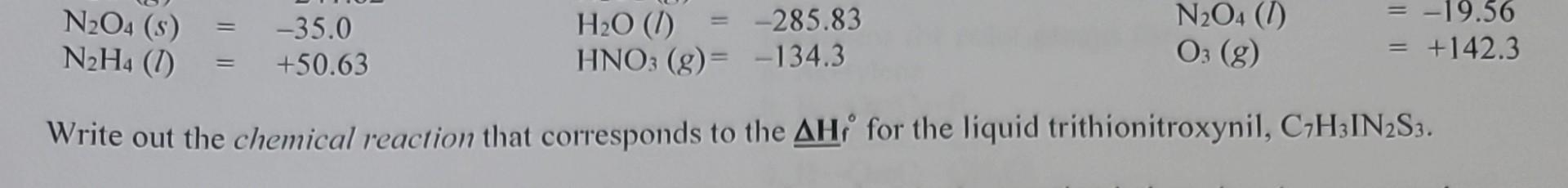
How do you go about this question? I'm not sure how I am supposed to know what the reactants are? Explain please.
N2O4(s)=35.0N2H4(l)=50.63H2O(l)=285.83HNO3(g)=134.3N2O4(l)O3(g)=19.56=+142.3 Write out the chemical reaction that corresponds to the H for the liquid trithionitroxynil, C7H3IN2S3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


