Question: how do you identify the intermolecular force found between in Br2 molecules 3.2 Consider the boiling points of the compounds in the table below. Promat
how do you identify the intermolecular force found between in Br2 molecules
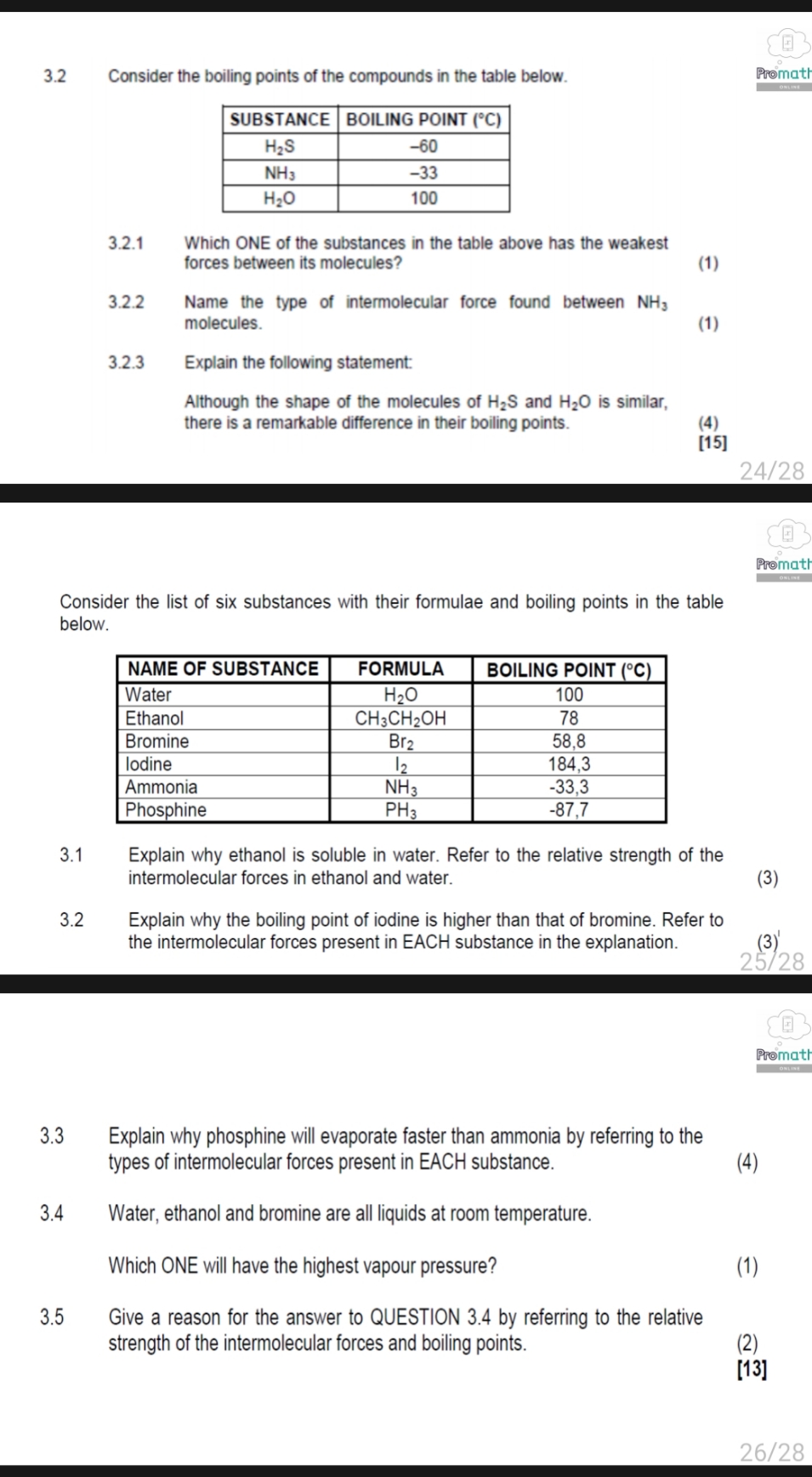
3.2 Consider the boiling points of the compounds in the table below. Promat SUBSTANCE BOILING POINT (C) H2S -60 NH -33 H20 100 3.2.1 Which ONE of the substances in the table above has the weakest forces between its molecules? (1) 3.2.2 Name the type of intermolecular force found between NH; molecules. (1) 3.2.3 Explain the following statement: Although the shape of the molecules of H2S and H2O is similar, there is a remarkable difference in their boiling points. (4) [15] 24/28 Promat Consider the list of six substances with their formulae and boiling points in the table below. NAME OF SUBSTANCE FORMULA BOILING POINT (C) Water H20 100 Ethanol CH3CH2OH 78 Bromine Br2 58,8 lodine 12 184,3 Ammonia NH 3 -33,3 Phosphine PHa -87,7 3.1 Explain why ethanol is soluble in water. Refer to the relative strength of the intermolecular forces in ethanol and water. (3) 3.2 Explain why the boiling point of iodine is higher than that of bromine. Refer to the intermolecular forces present in EACH substance in the explanation. (3) 25/28 Promat 3.3 Explain why phosphine will evaporate faster than ammonia by referring to the types of intermolecular forces present in EACH substance. (4) 3.4 Water, ethanol and bromine are all liquids at room temperature. Which ONE will have the highest vapour pressure? (1) 3.5 Give a reason for the answer to QUESTION 3.4 by referring to the relative strength of the intermolecular forces and boiling points. (2) [13] 26/28
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


