Question: How is my theoretical yield wrong? I have already altered the moles for the 85% phosphoric acid, I believe I have the correct answer now,
How is my theoretical yield wrong? I have already altered the moles for the 85% phosphoric acid, I believe I have the correct answer now, but I do not understand why my theoretical yield could be wrong
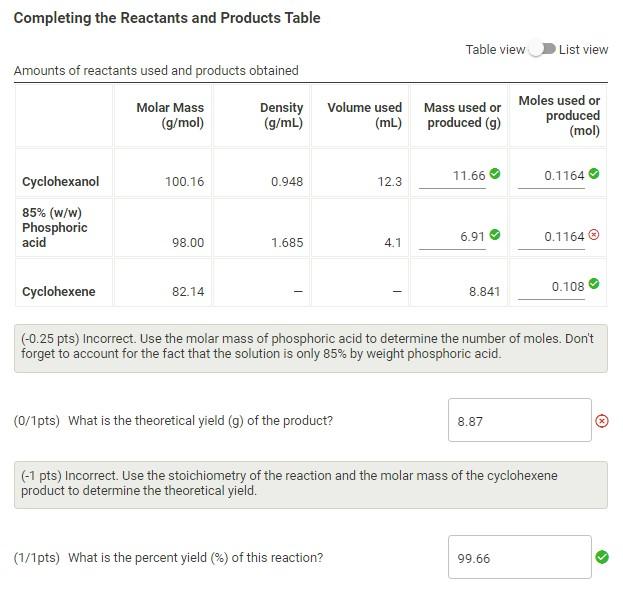
Completing the Reactants and Products Table Table view List view Amounts of reactants used and products obtained (0.25pts) Incorrect. Use the molar mass of phosphoric acid to determine the number of moles. Don't forget to account for the fact that the solution is only 85% by weight phosphoric acid. (0/1pts) What is the theoretical yield (g) of the product? (x) (-1 pts) Incorrect. Use the stoichiometry of the reaction and the molar mass of the cyclohexene product to determine the theoretical yield. (1/1pts) What is the percent yield (\%) of this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


