Question: How many electrons in an atom could have these sets of quantum numbers? n= 2 electrons n= 5,1 = 3 electrons n=6,t = 2, me
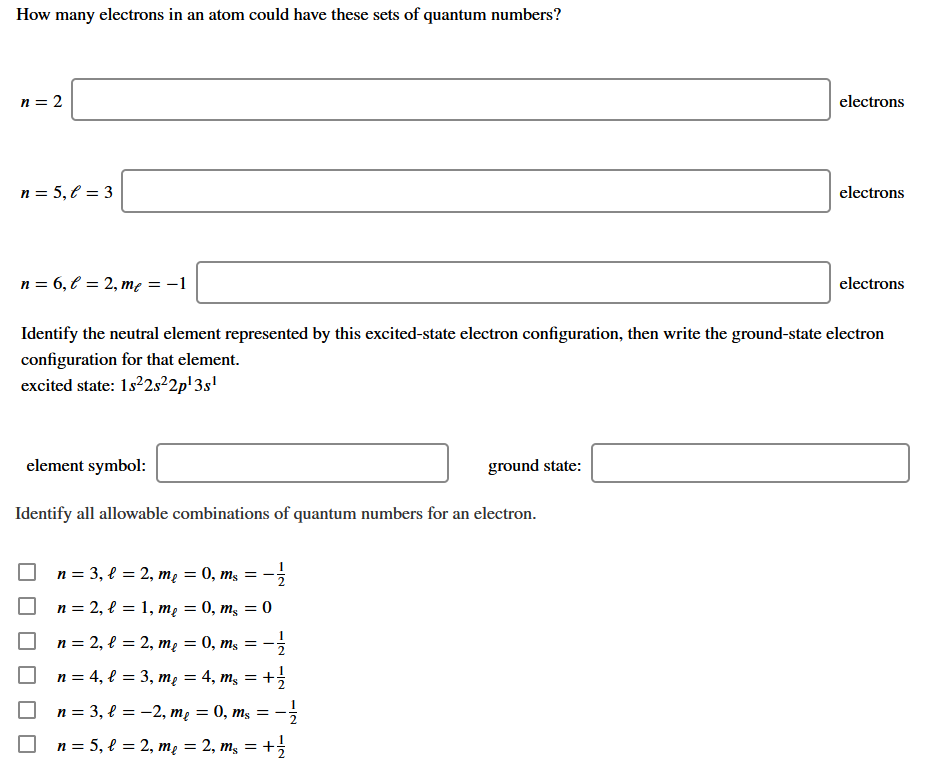
How many electrons in an atom could have these sets of quantum numbers? n= 2 electrons n= 5,1 = 3 electrons n=6,t = 2, me = -1 electrons Identify the neutral element represented by this excited-state electron configuration, then write the ground-state electron configuration for that element. excited state: 1s22s22p'3s! element symbol: ground state: Identify all allowable combinations of quantum numbers for an electron. = n=3, l = 2, me = 0, ms n=2, l = 1, me = 0, m = 0 n=2, l = 2, me = 0, ms = n= 4, f = 3, me = 4, ms = +1 n=3, l = -2, me = 0, ms = - :- n = 5, f = 2, me = 2, mg = +
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


