Question: How many significant figures should the answer to the following calculation have? 10.00 mL x 0.998765 Question 12 9 mL How would you express the
How many significant figures should the answer to the following calculation have? 10.00 mL x 0.998765 Question 12 9 mL How would you express the following number in scientific notation? Enter the number and exponent you would use. 0.00506 x 10^
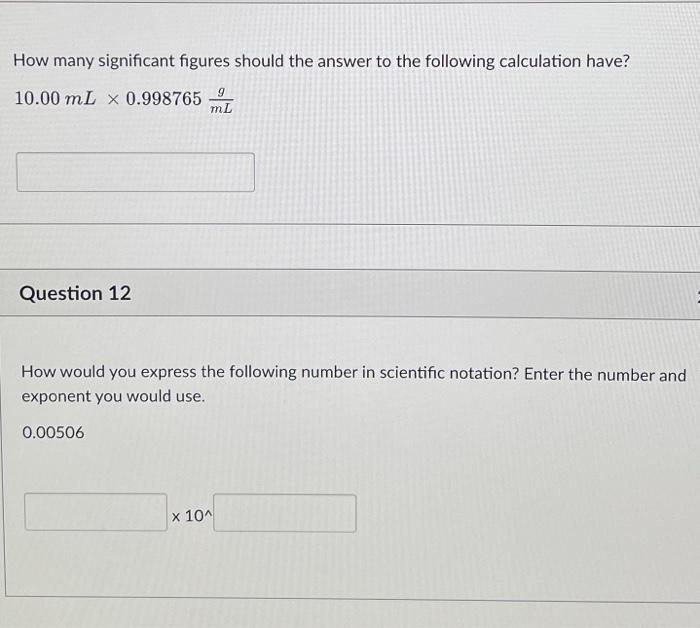
How many significant figures should the answer to the following calculation have? 10.00mL0.998765mLg Question 12 How would you express the following number in scientific notation? Enter the number and exponent you would use. 0.00506 10
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


