Question: How much dry solute would you take to prepare each of the following solutions from the dry solute and the solvent? 132gof0.220mNaNO3 Express your answer

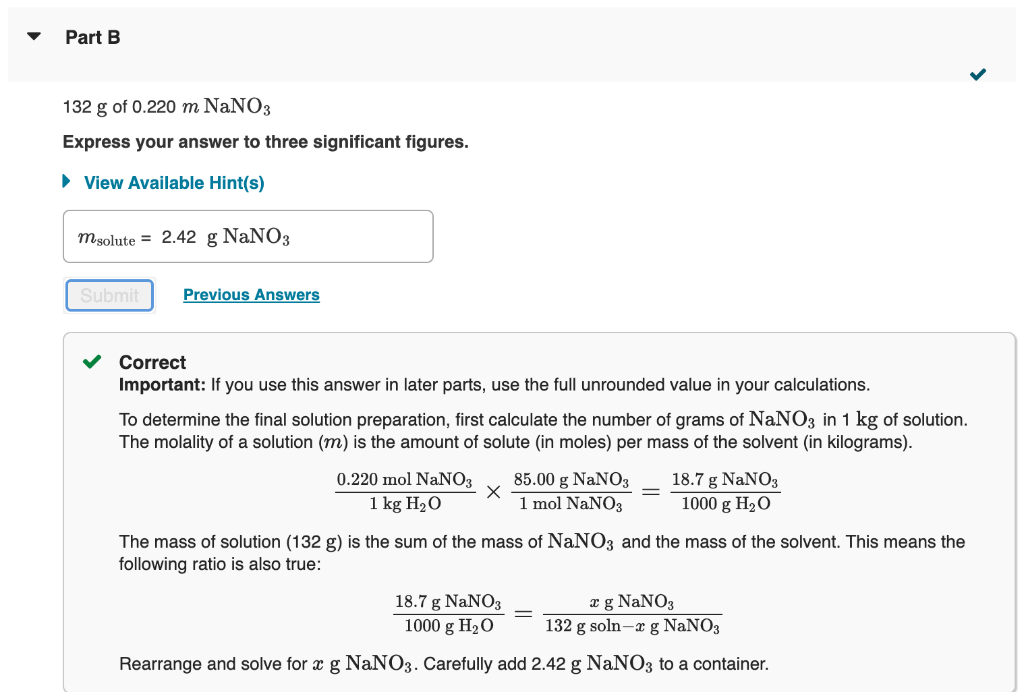
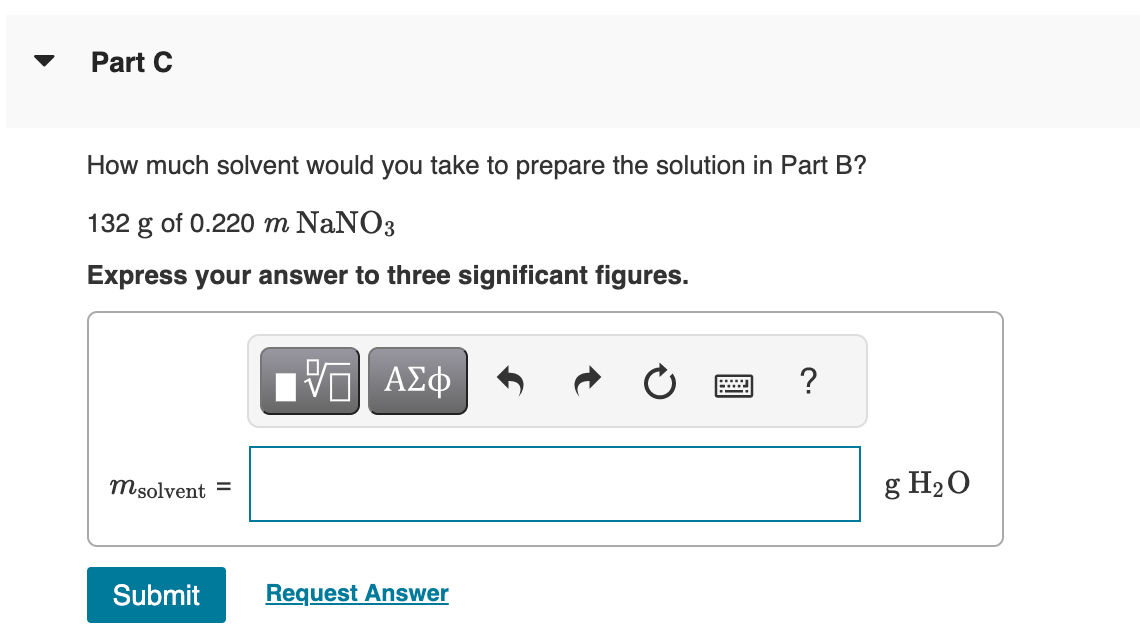
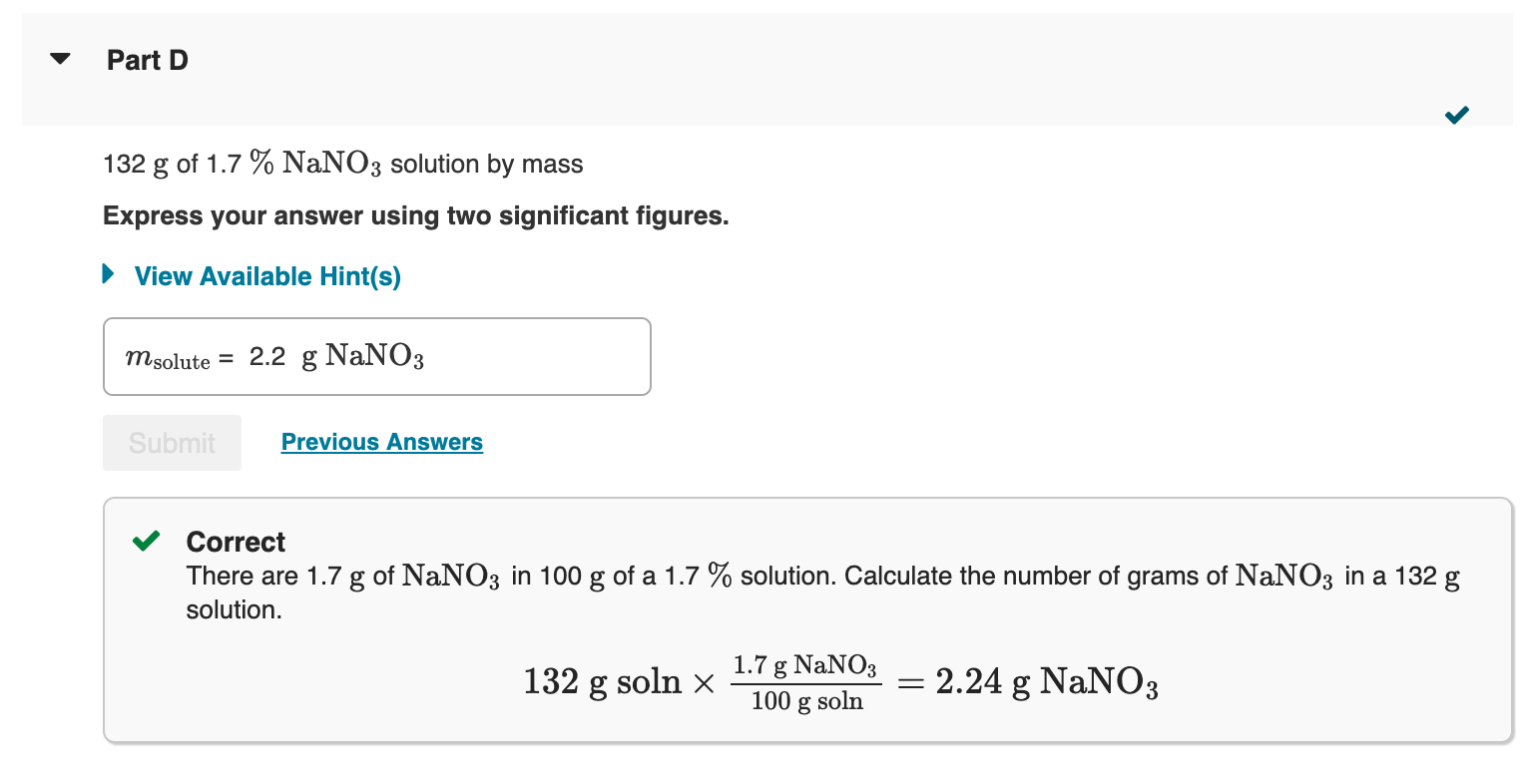
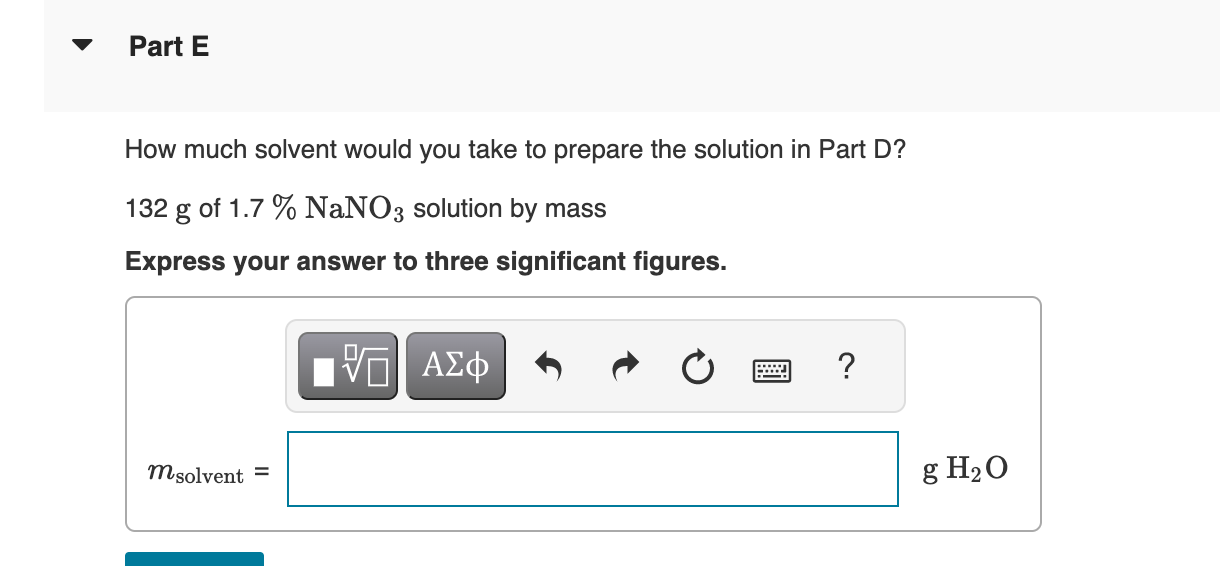
How much dry solute would you take to prepare each of the following solutions from the dry solute and the solvent? 132gof0.220mNaNO3 Express your answer to three significant figures. View Available Hint(s) Correct Important: If you use this answer in later parts, use the full unrounded value in your calculations. To determine the final solution preparation, first calculate the number of grams of NaNO3 in 1kg of solution. The molality of a solution (m) is the amount of solute (in moles) per mass of the solvent (in kilograms). 1kgH2O0.220molNaNO31molNaNO385.00gNaNO3=1000gH2O18.7gNaNO3 The mass of solution (132g) is the sum of the mass of NaNO3 and the mass of the solvent. This means the following ratio is also true: 1000gH2O18.7gNaNO3=132gsolnxgNaNO3xgNaNO3 How much solvent would you take to prepare the solution in Part B? 132gof0.220maNa3 Express your answer to three significant figures. 132g of 1.7%NaNO3 solution by mass Express your answer using two significant figures. Correct There are 1.7g of NaNO3 in 100g of a 1.7% solution. Calculate the number of grams of NaNO3 in a 132g solution. How much solvent would you take to prepare the solution in Part D? 132g of 1.7%NaNO3 solution by mass Express your answer to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


